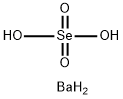
BARIUM SELENATE
$43.9 - $2609.84
- Product nameBARIUM SELENATE
- Cas No7787-41-9
- MFBaH4O4Se
- MW284.33
- EINECS232-113-8
- MDL NumberMFCD00046181
| Brand | Packaging | Price | Product name | |
|---|---|---|---|---|
|
American Custom Chemicals Corporation
ING0002453
|
10G | $1140.23 | BARIUM SELENATE |
|
|
American Custom Chemicals Corporation
ING0002453
|
50G | $2609.84 | BARIUM SELENATE |