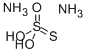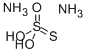Ammonium thiosulfate is an inorganic compound. It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether. And Ammonium thiosulfate is used in photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers. Fixation involves these chemical reactions (illustrated for silver bromide). Ammonium thiosulfate is also used for leaching of gold and silver. It works with presence of copper as a catalyst here. This process is a nontoxic alternative gold cyanidation. Ammonium thiosulfate can be used as a fertilizer. As suggested by some research studies it can be used as an additive to coal-waste mixtures to reduce formation of very dangerous dioxins and furans.
Methods of Manufacturing
Direct reaction of ammonium sulfite with sulfur. Besides,the ATS process, sulfur dioxide is absorbed from incinerated Claus tail gas in aqueous ammonia to produce ammonium sulfate and ammonium bisulfite in solution. The solution passes to a converter where it flows countercurrent to hydrogen sulfide. Ammonium thiosulfate forms /in solution and/ is concentrated to 60 wt%.