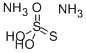Ammonium thiosulfate is an inorganic compound. It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether. And Ammonium thiosulfate is used in photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers. Fixation involves these chemical reactions (illustrated for silver bromide). Ammonium thiosulfate is also used for leaching of gold and silver. It works with presence of copper as a catalyst here. This process is a nontoxic alternative gold cyanidation. Ammonium thiosulfate can be used as a fertilizer. As suggested by some research studies it can be used as an additive to coal-waste mixtures to reduce formation of very dangerous dioxins and furans.
Methods of Manufacturing
Direct reaction of ammonium sulfite with sulfur. Besides,the ATS process, sulfur dioxide is absorbed from incinerated Claus tail gas in aqueous ammonia to produce ammonium sulfate and ammonium bisulfite in solution. The solution passes to a converter where it flows countercurrent to hydrogen sulfide. Ammonium thiosulfate forms /in solution and/ is concentrated to 60 wt%.
Ammonium thiosulfate exists as colorless, monoclinic or white crystals with ammonia like odor. It is very soluble in cold water; slightly soluble in acetone and insoluble in alcohol and ether. It is used to clean white metal; in lubricants for metal cold working, as an analytical reagent; fungicide, reducing agent; brightener in silver plating bath, and in hair waving preparations; fog screens, and cleaning compounds for zinc base die cast metals and it is used as a desiccant and defoliant in crop bearing plants. Inhalation of dust may irritate respiratory system. Contact with eyes or skin may cause irritation. Ingestion may be harmful.
1.https://pubchem.ncbi.nlm.nih.gov/compound/ammonium_thiosulfate#section=FDA-Requirements
2. https://en.wikipedia.org/wiki/Ammonium_thiosulfate
Ammonium thiosulfate is a white crystallinesolid with an ammonia odor. Molecular weight= 148.21;Freezing/Melting point= 150 C [decomposes ,50℃(solution); .100℃ (anhydrous crystals)]. HazardIdentification (based on NFPA-704 M Rating System):Health 1, Flammability 0, Reactivity 0. Highly soluble inwater.
white or colourless crystals
Ammonium thiosulfate is a white crystalline
solid with an ammonia odor.
Colorless, monoclinic crystal; hygroscopic; decomposes on heating above 100°C; density 1.679 g/cm3; very soluble in water (64 g/100 g at 20°C), insoluble in alcohol, and slightly soluble in acetone.
Ammonium thiosulfate finds application for leaching of gold and silver since it forms strong complexes. It is widely utilized as a photographic fixing agent, which acts quicklier than sodium thiosulfate fixers. It serves as a fertilizer. It acts as an additive to coal-waste mixtures to inhibit the formation of dangerous dioxins and furans. It is used as an analytical reagent in laboratories.
To clean "white" metal; in photography; in lubricants for metal cold-working.
AMMONIUM THIOSULFATE is a white crystalline solid. AMMONIUM THIOSULFATE is very soluble in water. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. AMMONIUM THIOSULFATE is used in photography, in chemical analysis, and for many other uses.
Hygroscopic. Very soluble in water.
AMMONIUM THIOSULFATE is sensitive to heat. AMMONIUM THIOSULFATE is incompatible with magnesium and aluminum powder. Mixtures with sodium chlorate can cause an exothermic reaction which can then decompose explosively. . Reacts with strong oxidizers such as chlorates, nitrates, and nitrites to release toxic ammonia, hydrogen sulfide, and sulfur trioxide gases. Will not polymerize. Ammonia, hydrogen sulfide, and oxides of nitrogen and oxides of sulfur may form in fires [USCG, 1999].
Inhalation of dust may irritate respiratory system. Ingestion could be harmful. Contact with eyes or skin may cause irritation.
Special Hazards of Combustion Products: Toxic ammonia, hydrogen sulfide, and oxides of nitrogen and sulfur may form in fires.
Ammonium thiosulphate, [(NH4)2S2O3 or ATS] is a
crystalline salt, the aqueous solution of which is
commonly used as a liquid fertilizer. Crystalline
ammonium thiosulphate contains 19% nitrogen and 43 %
sulphur and its aqueous solution contains 12% nitrogen
and 26 % sulphur.
Ammonium thiosulphate is compatible with nitrogen
and nitrogen-potassium solutions, which are neutral or
slightly acidic (pH 5.8). It is also used as a suspension:
As it is non-corrosive, it can be stored in steel or
aluminumdrums.
Ammonium thiosulphate is prepared by the reaction
of sulphur dioxide and aqueous ammonia, followed by a
further reaction with elemental sulphur.
ATS can be applied directly to soils or as a foliar spray
through sprinkler irrigation systems. When applied to soils,
ATS decomposes into ammonium sulphate and colloidal
sulphur. The ammonium sulphate is available immediately
to plants, while the sulphur gets oxidized over a period of
time to sulphate ion (SO42-) It is thus available to plants for
a longer time.
Ammonium thiosulphate also acts as a urease
inhibitor. When added to a urea-ammonium nitrate
solution, it inhibits urease activity for a month or so. In
industry, it is also used as a photographic fixing agent,
analytical reagent, fungicide and as a brightener in silverplating
baths.
Moderately toxic by ingestion.When heated to decomposition it emits toxic vapors ofNH4- and SOx.
Used as an agricultural chemical and
fungicide, metal lubricant; in cleaning metals; in photographic
chemicals, making other chemicals. A laboratory
reagent.
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Color Code—Green: General storage may beused. Prior to working with this chemical you should betrained on its proper handling and storage. Store in tightlyclosed containers in a cool, well-ventilated area away fromoxidizers, acids, water, or combustible materials.
UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous
material, Technical Name Required.
Contact with sodium chlorate may cause
a violent reaction. Corrodes brass, copper, and copperbased
metals.
Incinerate. It may be possible
to dispose of waste material at a municipal facility if
treated, neutralized and oxidized.