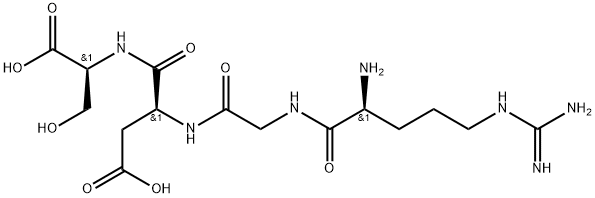
Fibronectin is an extracellular matrix protein which binds other proteins and interacts with cells, serving roles in cell adhesion, migration, and signaling. Like vitronectin and collagen, fibronectin contains a conserved tripeptide sequence, Arg-Gly-Asp (RGD) to associate with integrins on the cell surface. RGDS peptide is a tetrapeptide found on fibronectin, fibrinogen α, and von Willebrand factor, but not vitronectin or collagen. RGDS interacts with α5β1 and αVβ3 integrins. This tetrapeptide interferes with the attachment of cells to fibronectin-coated surfaces (Ki = 0.6 mM). It also blocks the attachment of certain pathogens to cells. RGDS peptide inhibits thrombin-induced binding of platelets to fibronectin, fibrinogen α, and von Willebrand factor (IC50 = ~10 μM). The interaction of RGDS peptide with cell surface integrins alters intracellular signaling in ways that are cell- and stimulus-specific.