-
순도시험
(1) 용상 : 이 품목 2g을 물 100mL에 녹일 때, 그 액은 무색으로서 그 탁도는 거의 징명 이하이어야 한다.
(2) 액성 : 이 품목의 수용액(1→100)의 pH는 5.6~6.1이어야 한다.
(3) 염화물 : 위 (1)의 액 20mL에 묽은질산 6mL를 가하여 이를 시험용액으로 하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.4mL에 대응하는 양 이하이어야 한다.
(4) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(5) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
-
확인시험
(1) 이 품목의 수용액(1→1,000) 5mL에 닌히드린용액(1→1,000) 1mL를 가하고 3분간 가열할 때 자색을 나타낸다.
(2) 이 품목의 수용액(1→100)은 선광성이 없다.
(3) 이 품목 25mg을 무수황산동을 포화한 황산 1mL에 가하면 황색을 나타낸다.
(4) 이 품목의 수용액(1→100) 2mL에 수산화나트륨용액(1→25) 2mL를 가하여 잘 흔들어 섞고 니트로프루시드나트륨시액 0.3mL를 가하여 다시 잘 흔들어 섞은 다음 1~2분간 방치하고 이에 염산(1→10) 4mL를 가하여 잘 흔들어 섞으면 적자색을 나타낸다.
-
정량법
이 품목을 건조한 다음 약 0.6g을 정밀히 달아 물에 녹여 100mL로 하고 그 중 50mL를 공전플라스크에 넣고 물 50mL, 제이인산칼륨 5g, 제일인산칼륨 2g 및 요오드 칼륨 2g을 가하여 흔들어 섞어 녹인다. 다음 0.1N 요오드용액 50mL를 가하여 밀전하여 잘 흔들어 섞고 30분간 방치한 다음 과잉의 요오드를 0.1N 치오황산나트륨용액으로 적정한다(지시약 : 전분시액). 따로 같은 방법으로 공시험을 한다.
0.1N 요오드용액 1mL = 7.461mg C5H11O2NS
-
강열잔류물
이 품목의 강열잔류물은 0.1% 이하이어야 한다.
-
개요
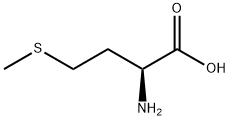
Methionine is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the initiation codon AUG which indicates mRNA's coding region where translation into protein begins.
-
화학적 성질
White crystalline powder
-
출처
High levels of methionine can be found in eggs, sesame seeds, Brazil nuts, fish, meats and some other plant seeds; methionine is also found in cereal grains. Most fruits and vegetables contain very little of it. Most legumes are also low in methionine. Racemic methionine is sometimes added as an ingredient to pet foods.
-
용도
DL-Methionine is sometimes given as a supplement to dogs; it helps keep dogs from damaging grass by reducing the pH of the urine.
Methionine is allowed as a supplement to organic poultry feed under the US certified organic program.
-
제조 방법
By addition of methanethiol to acrolein; by chemical conversion of methylthiopropionic aldehyde.
-
정의
ChEBI: A sulfur-containing amino acid that is butyric acid bearing an amino substituent at position 2 and a methylthio substituent at position 4.
-
Biosynthesis
As an essential amino acid, methionine is not synthesized de novo in humans, who must ingest methionine or methioninecontaining proteins. In plants and microorganisms, methionine is synthesized via a pathway that uses both aspartic acid and cysteine. First, aspartic acid is converted via β-aspartyl-semialdehyde into homoserine, introducing the pair of contiguous methylene groups. Homoserine converts to O-succinyl homoserine, which then reacts with cysteine to produce cystathionine, which is cleaved to yield homocysteine. Subsequent methylation of the thiol group by folates affords methionine. Both cystathionine-γ-synthase and cystathionine- β-lyase require pyridoxyl-5′-phosphate as a cofactor, whereas homocysteine methyltransferase requires vitamin B12 as a cofactor.
-
Biological Functions
Together with cysteine, methionine is one of two sulfurcontaining proteinogenic amino acids. Its derivative S-adenosyl methionine (SAM) serves as a methyl donor. Methionine is an intermediate in the biosynthesis of cysteine, carnitine, taurine, lecithin, phosphatidylcholine, and other phospholipids. Improper conversion of methionine can lead to atherosclerosis.
This amino acid is also used by plants for synthesis of ethylene. The process is known as the Yang Cycle or the methionine cycle.
Methionine is one of only two amino acids encoded by a single codon (AUG) in the standard genetic code (tryptophan, encoded by UGG, is the other). The codon AUG is also the most common eukaryote "Start" message for a ribosome that signals the initiation of protein translation from mRNA when the AUG codon is in a Kozak consensus sequence. As a consequence, methionine is often incorporated into the N-terminal position of proteins in eukaryotes and archaea during translation, although it can be removed by post-translational modification. In bacteria, the derivative Nformylmethionine is used as the initial amino acid.
-
일반 설명
DL-Methionine is an essential amino acid containing sulphur. Methionine consists of an asymmetric carbon and exists as D (dextrogyre) and L (levogyre) optical isomers. The L-methionine is considered as biologically active. The racemic mixture of D and L-isomers forms DL-methionine, which is the commercially available methionine.
-
Safety Profile
Moderately toxic by
ingestion and other routes. An experimental
teratogen. Experimental reproductive
effects. When heated to decomposition it
emits toxic fumes of SOx and NOx. See also
1-METHIONINE.
-
Purification Methods
Crystallise it from hot water or EtOH. Also purify it by dissolving it in H2O and passing through an Amberlite IR-120 resin (NH4+ form). The eluate is concentrated and then passed through Amberlite IR-4B resin, and this eluate is evaporated to dryness. The residue is washed with EtOH, then Me2CO, dried and recrystallised from aqueous EtOH (colourless plates) [Baddiley & Jamieson J Chem Soc 4283 1954]. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2125 1961, Beilstein 4 IV 3190.]