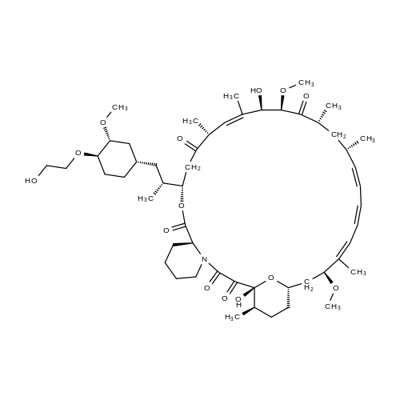
エベロリムス
化学名:エベロリムス
CAS番号.159351-69-6
英語名:Everolimus
CBNumberCB9502411
MFC53H83NO14
MW958.22
MOL File159351-69-6.mol
别名
エベロリムス(異性体混合物)
エベロリムス 溶液
エベロリムス
more
エベロリムス物理性質
| 融点 | NA |
| 沸点 | 998.7±75.0 °C(Predicted) |
| 比重(密度) | 1.18±0.1 g/cm3(Predicted) |
| 闪点 | 2℃ |
| 貯蔵温度 | -20°C |
| 溶解性 | DMSO (最大 100mg/ml) またはエタノール (最大 100mg/ml) に可溶。 |
| 外見 | 個体 |
| 酸解離定数(Pka) | 10.40±0.70(Predicted) |
| 色 | 白い |
| 水溶解度 | ジメチルスルホキシド、エタノール、クロロホルムに可溶。水にわずかに溶ける。 |
| 安定性: | 吸湿性 |
| InChIKey | HKVAMNSJSFKALM-GKUWKFKPSA-N |
| 主な危険性 | T,Xn,F |
| Rフレーズ | 48/25-36-20/21/22-11 |
| Sフレーズ | 45-36/37-26-16 |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 2 |
| F | 10 |
| HSコード | 29349990 |
| 有毒物質データの | 159351-69-6(Hazardous Substances Data) |

