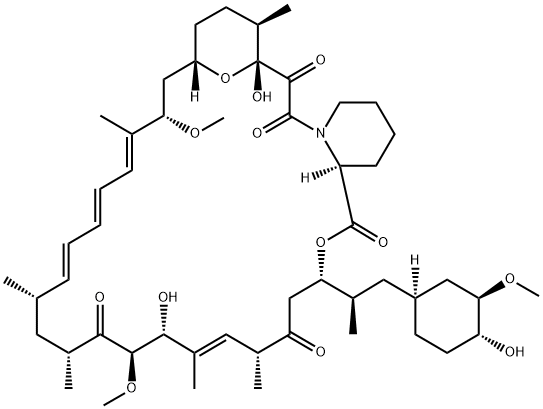
(-)-ラパマイシン
化学名:(-)-ラパマイシン
CAS番号.53123-88-9
英語名:Rapamycin
CBNumberCB0275190
MFC51H79NO13
MW914.17
MOL File53123-88-9.mol
别名
シロリムス
ラパマイシン
(-)-ラパマイシン
more
(-)-ラパマイシン物理性質
| 融点 | 183-185°C |
| 比旋光度 | D25 -58.2° (methanol) |
| 沸点 | 799.83°C (rough estimate) |
| 比重(密度) | 1.0352 (rough estimate) |
| 蒸気圧 | 0.56 hPa ( 20 °C) |
| 屈折率 | 1.5280 (estimate) |
| 闪点 | 87 °C |
| 貯蔵温度 | -20°C |
| 溶解性 | エタノール:ソリュブル2MM |
| 酸解離定数(Pka) | 10.40±0.70(Predicted) |
| 外見 | 解決 |
| 色 | 無色~黄色 |
| 水溶解度 | 50 mg/ml DMSO または 25 mg/ml メタノールに溶解。エタノール、ジメチルスルホキシド、ジメチルホルムアミドに可溶。水に溶けません。 |
| Sensitive | Moisture Sensitive/Light Sensitive/Hygroscopic |
| Merck | 14,8114 |
| 安定性: | 購入日から2年間安定です。この DMSO またはエタノール溶液は、-20°C で 2 か月間保存できます。 |
| CAS データベース | 53123-88-9(CAS DataBase Reference) |
| 主な危険性 | Xi,Xn,F |
| Rフレーズ | 36/38-36-20/21/22-11 |
| Sフレーズ | 22-24/25-37/39-26-36/37-16 |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 2 |
| RTECS 番号 | VE6250000 |
| 自然発火温度 | 301 °C |
| HSコード | 29349990 |
| 有毒物質データの | 53123-88-9(Hazardous Substances Data) |
| 毒性 | LD50 in mice (mg/kg): 600 i.p.; >2,500 orally (Vézina) |
