-
解説
無水物は立方晶系.密度2.2 g cm-3(4 ℃).HCl,HNO3に可溶.水,酢酸に難溶.水ではKsp 2.5×10-9 mol2 dm-6(25 ℃).カルシウムイオンおよびシュウ酸イオンの定性・定量分析に利用される.陶器のうわぐすり,希土類分離の担体などに用いられる.
森北出版「化学辞典(第2版)
-
用途
カルシウムイオンおよびシュウ酸イオンの定性・定量分析に利用される.陶器のうわぐすり,希土類分離の担体などに用いられる.
-
製造
シュウ酸カルシウム,鉱石として,天然にヒューエライト(whewellite)(一水和物),ウェデライト(weddellite)(二水和物)がある.カルシウム塩の水溶液にシュウ酸アンモニウムを加えると一水和物が得られる.これを約250 ℃ で乾燥すると無水物が得られる.二水和物は正方晶系,一水和物は単斜晶系であり,Caは8個のO原子で正方ねじれプリズム型に囲まれている.Ca-O2.4~2.5 Å.
-
説明
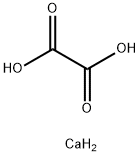
Calcium oxalate (in archaic terminology, oxalate of lime) is a chemical compound that forms envelope-shaped crystals, known in plants as raphides. A major constituent of human kidney stones, the chemical is also found in beerstone, a scale that forms on containers used in breweries. Its chemical formula is CaC2O4 or Ca (COO)2.
-
化学的特性
Calcium oxalate is white precipitate, insoluble in weak acids, but soluble in strong acids, formed by reaction of soluble calcium salt solution and ammonium oxalate solution. Solubility at 18 °C 0.0056 g anhydrous salt per liter of saturated solution.
-
物理的性質
Most crystals look like a 6 sided prism and often look like a pointed picket from a wooden fence. More than 90 % of the crystals in a urine sediment will have this type of morphology. These other shapes are less common than the 6 sided prism, however it is important to be able to quickly identify them in case of emergency.
-
天然物の起源
Many plants are accumulating calcium oxalate (it has been reported in 1000 genera of tree ). The calcium oxalate accumulation is linked to the detoxification of calcium (Ca2+) in the plant.
Calcium oxalate is a poisonous substance that can produce sores and numbing on ingestion and could even be fatal.
The poisonous plant dumb cane (Dieffenbachia) contains the substance and on ingestion can prevent speech and be suffocating. It is also found in rhubarb (in large quantities in the leaves) and in species of Oxalis, Araceae, taro, kiwifruit, tea leaves, agaves, and Alocasia and in spinach in varying amounts. Insoluble calcium oxalate crystals are found in plant stems, roots, and leaves and produced in idioblasts. Calcium oxalate, as ' beer stone ', is a brownish precipitate that tends to accumulate within vats, barrels and other containers used in the brewing of beer.
-
定義
ChEBI: The calcium salt of oxalic acid, which in excess in the urine may lead to formation of oxalate calculi (kidney stones).
-
主な応用
Calcium oxalate is used in the manufacture of ceramic glazes.
-
一般的な説明
Purity based on trace metal analysis
-
健康ハザード
Even a small dose of calcium oxalate is enough to cause intense sensations of burning in the mouth and throat, swelling, and choking that could last for up to two weeks . In greater doses it can cause severe digestive upset, breathing difficulties, coma or even death. Recovery from severe oxalate poisoning is possible, but permanent liver and kidney damage may have occurred.
The stalks of plants in the Dieffenbachia genus produce the most severe oxalate reactions. The needle - like oxalate crystals produce pain and swelling when they contact lips, tongue, oral mucosa, conjunctiva, or skin. Edema primarily is due to direct trauma from the needle-like crystals and, to a lesser extent, by other plant toxins (e.g., bradykinins, enzymes).
Depending on the plant ingested, mild (Elephant Ear Colocasia esculenta) to more severe (Jack in the Pulpit, Arisaema) can cause compromised airways. One bite on the Arisaema seed pod will result in immediate swelling and burning. It will take over 12 hours for the swelling to subside .
4 – 1 - Treatment
Medication administered at the emergency room may include diphenhydramine, epinephrine, or famotidine, all intravenously. Although this most likely will be a localized reaction, it will be treated by the ER as an anaphylactic reaction.
-
人間への毒性
しゅう酸カルシウムは、皮膚や粘膜に対し強い刺激性・腐食性を持ちます。手や目に付着しないよう、や保護めがね等の保護具を着用します。
しゅう酸カルシウムが皮膚に付着した場合は、流水ですすぎながら石鹸でよく洗います。石鹸を用いるのは、 pH を上げることでしゅう酸カルシウムの溶解度が上がり、洗い流しやすくなるためです。
眼に入った場合は、しゅう酸カルシウムの結晶が目から流れ出やすいように、顔を横にした状態で流水で洗浄します。このとき、まぶたを指で広げて、眼球をゆっくり動かしながら、まぶたの裏までよく洗うようにします。目に刺激感が残る場合は医師の診察が必要です。
-
使用用途
しゅう酸カルシウム単体の使用用途は、カルシウムイオンやしゅう酸イオンの定性・定量分析の標準溶液、陶器の釉薬 (うわぐすり) などです。しゅう酸カルシウムは、しゅう酸を製造する際の中間体として発生します。
しゅう酸カルシウムにを加えると、が析出して、しゅう酸イオンが遊離します。硫酸カルシウムを濾過で除去し、濾液を蒸発させるとしゅう酸の結晶が析出します。
-
特徴
しゅう酸カルシウムはカルシウムのしゅう酸塩であり、化学式はCaC2O4です。水が配位結合して、一水和物や二水和物を形成することがあります。
実験用試薬などでは、一水和物の状態で販売されている場合もあるため、実験に用いる際はラベル表示の確認が必要です。なお、しゅう酸カルシウムの水和物を200℃に加熱すると、結晶種を失って無水物となります。
しゅう酸カルシウムの基本的な特性 (分子量、比重、溶解性) は以下の通りです。
- 分子量:146.11
- 密度:2.2g/cm3
- 溶解性:水、にほとんど溶けない。希塩酸、希硫酸に溶ける。