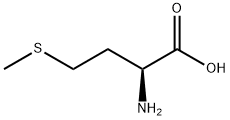
DL-メチオニン
化学名:DL-メチオニン
CAS番号.59-51-8
英語名:DL-Methionine
CBNumberCB6208758
MFC5H11NO2S
MW149.21
MOL File59-51-8.mol
别名
DL-メチオニン
(±)-2-アミノ-4-(メチルチオ)酪酸
rac-(R*)-2-アミノ-4-(メチルチオ)ブタン酸
more
DL-メチオニン物理性質
| 融点 | 284 °C (dec.)(lit.) |
| 比旋光度 | -1~+1°(D/20℃)(c=8,HCl) |
| 沸点 | 306.9±37.0 °C(Predicted) |
| 比重(密度) | 1.34 |
| FEMA | 3301 | D,L-METHIONINE |
| 屈折率 | 1.5216 (estimate) |
| 貯蔵温度 | 2-8°C |
| 溶解性 | 1M HCl: 20°C で 0.5 M、透明、無色 |
| 外見 | 結晶または結晶性粉末 |
| 酸解離定数(Pka) | 2.13(at 25℃) |
| 色 | 白い |
| 臭い (Odor) | 弱亜硫酸性 |
| においのタイプ | 酸性の |
| 由来生物 | synthetic |
| 光学活性 (optical activity) | [α]/D, c = 5 in 5 M HCl (inactive) |
| 水溶解度 | 2.9g/100mL(20℃) |
| Merck | 14,5975 |
| JECFA Number | 1424 |
show more
| 主な危険性 | Xi |
| Rフレーズ | 33-36/37/38 |
| Sフレーズ | 24/25-36-26 |
| WGK Germany | 2 |
| RTECS 番号 | PD0457000 |
| F | 10-23 |
| TSCA | Yes |
| HSコード | 29304090 |
| 化審法 | (2)-1254, (2)-1467 届出不要化学物質 |