-
性質
過酢酸はやエーテル等によく溶けます。水と反応して、と過酸化水素に分解されます。融点は0.1°C、沸点は105°Cです。
過酢酸はエタンペルオキソ酸とも呼ばれます。過酸 (英: peroxy acid) の1種で、オキソ酸におけるヒドロキシ基 (−OH) がヒドロペルオキシド基 (−O−OH) に変化した構造を有しています。
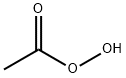
分子式はC2H4O3、示性式はCH3COO2H、分子量は76.05です。
-
解説
酢酸のカルボキシル基が過酸化物型に変わった化合物,CH3-C(=O)OOH.酸化剤として,とくにエポキシドの合成に使われる.
-
用途
ポリエステル型樹脂の低温重合触媒、殺菌剤、有機合成用酸化剤
-
効能
殺菌消毒薬
-
商品名
アセサイド (サラヤ); アセサイドMA (サラヤ); エスサイド (富士フイルム富山化学)
-
説明
Since the early 1900s, chlorine has been used as a water disinfectant.
It was favored by water and wastewater industries for
disinfection until several harmful disinfection by-products were
discovered in chlorinated water. Studies were done to find and
eliminate disinfection byproduct precursors and look for an
alternative disinfectant, which turned out to be peracetic acid, or
PAA. Peracetic acid is a chemical product belonging to peroxide
compounds such as hydrogen peroxide. However, unlike
hydrogen peroxide, it is a more potent antimicrobial agent.
Peracetic acid has high germicidal efficiency and sterilizing
capability, and its degradation residuals are not dangerous to
the environment or toxic to human health. Until 1960,
peracetic acid was of special interest to the food processing
industry and actually was considered the only agent able to
replace glutaraldehyde in the sterilization of surgical, medical,
and odontoiatry instruments. The actual core medical applications
of peracetic acid are its potent antimicrobial action, also at
low temperatures, and the total absence of toxic residuals.
-
化学的特性
colourless liquid with an acrid odour
-
使用
Peroxyacetic acid is used as an epoxidizingagent, for bleaching, as a germicide and fungicide, and in the synthesis of pharmaceuticals.Its solution Dialox is used as a cleansing andsterilizing agent in the reuse of highly permeable dialyzers. Turcic et al. (1997) have reported the efficacy of peroxyacetic acid asa local antiseptic in healing war wounds.Oxidative degradation of polynuclear aromatic hydrocarbons by peroxy acid in contaminated soils has been effectively achieved(N’Guessan et al. 2004).
-
調製方法
Peracetic acid (PAA) is a mixture of acetic acid (CH3COOH)
and hydrogen peroxide (H2O2) in an aqueous solution. It is a
very strong oxidizing agent and has stronger oxidation
potential than chlorine or chlorine dioxide. Liquid, clear,
and colorless with no foaming capability. It has a strong
pungent acetic acid odor, and the pH is acid . Peracetic acid is produced by reacting acetic acid and hydrogen peroxide. The reaction is allowed to continue
for up to 10 days in order to achieve high yields of product.
Additional methods of preparation involve the oxidation of
acetaldehyde or alternatively as an end product of the reaction
of acetic anhydride, hydrogen peroxide, and sulfuric
acid. Another method involves the reaction of tetraacetylethylenediamine
(TAED) in the presence of an alkaline hydrogen peroxide solution.
-
一般的な説明
Colorless liquid with a strong, pungent acrid odor. Used as a bactericide and fungicide, especially in food processing; as a reagent in making caprolactam and glycerol; as an oxidant for preparing epoxy compounds; as a bleaching agent; a sterilizing agent; and as a polymerization catalyst for polyester resins.
-
空気と水の反応
Soluble in water
-
反応プロフィール
Self-reactive. Peracids should be handled only in small quantities and with extreme care when pure or very concentrated. Organic peracids, such as Peroxyacetic acid, are so unstable that they may explode during distillation, even under reduced pressure [NFPA 1991].
-
健康ハザード
Peroxyacetic acid is a severe irritant to theskin and eyes. It can cause severe acid burns.Irritation from 1 mg was severe on rabbits’eyes. Its toxicity is low. The toxicologicalroutes of entry to the body are inhalation,ingestion, and skin contact. The toxicity dataare as follows (NIOSH 1986):LC50 inhalation (rats): 450 mg/m3
LD50 oral (mice): 210 mg/kg
LD50 oral (guinea pigs): 10 mg/kg
Its toxicity in humans should be very low,and a health hazard may arise only fromits severe irritant action. Studies on miceshowed that it caused skin tumors at the siteof application. Its carcinogenicity on humansis not reported. No exposure limit is set forperoxyacetic acid in air.
-
火災危険
Decomposes violently at 230F. When heated to decomposition, Peroxyacetic acid emits acrid smoke and fumes. Runoff to sewer may create a fire or explosion hazard. Powerful oxidizer. Isolate from other stored material, particularly accelerators, oxidizers, and organic or flammable materials. Avoid shock and heat. Hazardous polymerization may not occur.
-
燃焼性と爆発性
Peracetic acid explodes when heated to 110 °C, and the pure compound is extremely
shock sensitive. Virtually all peracids are strong oxidizing agents and decompose
explosively on heating. Moreover, most peracids are highly flammable and can
accelerate the combustion of other flammable materials if present in a fire. Fires
involving peracetic acid can be fought with water, dry chemical, or halon
extinguishers. Containers of peracetic acid heated in a fire may explode.
-
使用用途
過酢酸には、酢酸へ分解する際に発生する酸素ラジカルにより、芽胞菌を含む多種多様な微生物を障害する働きがあります。このため、菌・真菌・ウイルスといった幅広い病原微生物に対して、殺菌剤として利用されています。
医療分野においては、内視鏡や透析機器の医療用消毒剤としても使用可能です。飲食分野では、「飲料工場におけるペットボトルやプラスチックキャップ等の殺菌」「野菜・果物の微生物制御」「鶏・牛・豚肉の表面殺菌剤」としても、広く用いられています。
他にも、二重結合のエポキシ化 (酸化剤) 、漂白剤などにも用いることが可能です。
-
农业用途
Fungicide, Herbicide, Nematicide, Rodenticide,
Microbiocide: This compound is used as bactericide and fungicide,
especially in food processing, a reagent in making caprolactam
and glycerol; an oxidant for preparing epoxy compounds;
a bleaching agent; a sterilizing agent; and a polymerization
catalyst for polyester resins. Not approved for use in EU
countries. Registered for use in the U.S. and Canada.
-
製品名
DESOXON 1®; ESTOSTERIL®; OSBON
AC®; OXYMASTER®; PROXITANE®
-
安全性プロファイル
Poison by ingestion. Moderately toxic by inhalation and skin contact. A corrosive eye, sktn, and mucous membrane irritant. Questionable carcinogen with experimental tumorigenic data by skin contact. Flammable liquid. Severe explosion hazard when exposed to heat or by spontaneous chemical reaction. Explodes violently at 1 10°C. A powerful oxidizing agent. Explosive reaction with acetic anhydride, 5-p-chlorophenyl-2,2-dimethyl-3hexanone. Violent reaction with ether solvents (e.g., tetrahydrofuran, diethyl ether), metal chloride solutions (e.g., calcium chloride, potassium chloride, sodium chloride), olefins, organic matter. Dangerous; keep away from combustible materials. When heated to decomposition it emits acrid smoke and irritating fumes. To fight fire, use water, foam, CO2. Used as a polymerization initiator, curing agent, and cross-linhng agent. See also PEROXIDES, ORGANIC.
-
概要
過酢酸とは、刺激臭のある無色の液体の過カルボン酸です。
におよびを加えて、蒸留することで生成されます。酢酸コバルトの存在下、または、紫外線照射下において、と酸素を混合することによって、生成する方法もあります。
110℃に加熱すると爆発しますが、希薄溶液の状態では非常に安定です。また、皮膚などを腐食するため、取り扱いには注意が必要です。通常、・過酸化水素・過酢酸の平衡混合物として存在しています。
-
環境運命予測
Routes and pathways, and relevant physicochemical properties
(e.g., solubility, Pow, Henry constant,.)
Melting point ? �0.2 °C.
Log Kow ? �1.07.
Solubility: very soluble in ether, sulfuric acid, and ethanol;
miscible with water 1.0 × 106 mg l�1 at 25 °C.
Henry’s law constant ? 2.14 × 10�6 atm-m3 mol�1 at 25 °C
Environmental persistency (degradation/speciation)
Peracetic acid is formed naturally in the environment through
a series of photochemical reactions involving formaldehyde and
photo oxidant radicals. The pKa of peracetic acid is 8.2, indicating
that this compound exists partially in anion form in the environment,
and anions generally do not adsorb more strongly to
soils containing organic carbon and clay than their neutral counterparts.
It degrades in the environment very quickly but has no
potential to bioaccumulate. Its ultimate fate in the environment is
in the basic molecules of carbon dioxide, oxygen, and water.
Bioaccumulation and biomagnification
An estimated BCF of 3 was calculated in fish for peracetic acid,
using an estimated log Kow of -1.07 and a regression-derived
equation. The BCF suggests that the potential for bioconcentration
in aquatic organisms is low.
-
貯蔵
Reactions involving large quantities
of peracids should be carried out behind a safety shield. Peracetic acid should be
used only in areas free of ignition sources and should be stored in tightly sealed
containers in areas separate from oxidizable compounds and flammable substances.
Other commonly available peracids, such as perbenzoic acid and m-chloroperbenzoic
acid (MCPBA), are less toxic, less volatile, and more easily handled than peracetic
acid.
-
不和合性
Peracids such as peracetic acid are strong oxidizing agents and react exothermically
with easily oxidized substrates. In some cases the heat of reaction can be sufficient to
induce ignition, at which point combustion is accelerated by the presence of the
peracid. Violent reactions may potentially occur, for example, with ethers, metal
chloride solutions, olefins, and some alcohols and ketones. Shock-sensitive peroxides
may be generated by the action of peracids on these substances as well as on
carboxylic anhydrides. Some metal ions, including iron, copper, cobalt, chromium,
and manganese, may cause runaway peroxide decomposition. Peracetic acid is also
reportedly sensitive to light.
-
廃棄物の処理
Excess peracetic acid and waste material containing this substance should be placed
in an appropriate container, clearly labeled, and handled according to your
institution's waste disposal guidelines. Peracids may be incompatible with other
flammable mixed chemical waste; for example, shock-sensitive peroxides can be
generated by reaction with some ethers such as THF and diethyl ether.