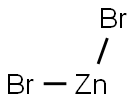ChemicalBook > CAS DataBase List > Zinc bromide
Zinc bromide
Zinc bromide
- CAS No.7699-45-8
- Chemical Name:Zinc bromide
- CBNumber:CB9680793
- Molecular Formula:Br2Zn
- Formula Weight:225.2
- MOL File:7699-45-8.mol
Zinc bromide Property
- Melting point 394 °C (lit.)
- Boiling point ~670 °C/1 atm (lit.)
- Density 4.22
- refractive index 1.5452
- Flash point 650°C
- storage temp. Store below +30°C.
- solubility Soluble in alcohol, ether, acetone and tetrahydrofuran.
- form beads
- Specific Gravity 4.2012
- color White to pale cream
- PH 4 (H2O, 20℃)(saturated solution)
- Water Solubility soluble, 447 g/100 mL (20 ºC)
- Sensitive Hygroscopic
- Merck 14,1029
- Stability Stable. Forms explosive mixtures with sodium and potassium. Protect from moisture.
- CAS DataBase Reference 7699-45-8(CAS DataBase Reference)
- EWG's Food Scores 1
- FDA UNII OO7ZBU9703
- NIST Chemistry Reference Zinc dibromide(7699-45-8)
- EPA Substance Registry System Zinc bromide (7699-45-8)
- UNSPSC Code 12352302
- NACRES NA.23
Safety
- Hazard Codes :C,N
- Risk Statements :34-50/53-50-51/53-43-22
- Safety Statements :26-36/37/39-45-60-61-29
- RIDADR :UN 3260 8/PG 3
- WGK Germany :3
- RTECS :ZH1150000
- F :3-21
- TSCA :Yes
- HS Code :2827 59 00
- HazardClass :8
- PackingGroup :II
- Hazardous Substances Data :7699-45-8(Hazardous Substances Data)
-
NFPA 704:
0 3 1
-
Symbol(GHS)



- Signal wordDanger
- Hazard statements H302-H314-H317-H411
- Precautionary statements P273-P280-P305+P351+P338-P310
Zinc bromide Price
More Price(22)
- Brand: Sigma-Aldrich(India)
- Product number: 8.18631
- Product name : Zinc bromide
- Purity: anhydrous for synthesis
- Packaging: 5G
- Price: ₹3360
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.18631
- Product name : Zinc bromide
- Purity: anhydrous for synthesis
- Packaging: 250G
- Price: ₹10640
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.18631
- Product name : Zinc bromide
- Purity: anhydrous for synthesis
- Packaging: 1KG
- Price: ₹27710
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich
- Product number: 451398
- Product name : Zinc bromide
- Purity: AnhydroBeads?, ?10?mesh, 99.999% trace metals basis
- Packaging: 5G
- Price: ₹5650.65
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 451398
- Product name : Zinc bromide
- Purity: AnhydroBeads?, ?10?mesh, 99.999% trace metals basis
- Packaging: 5G
- Price: ₹5650.65
- Updated: 2022/06/14
- Buy: Buy
Zinc bromide Chemical Properties,Usage,Production
-
Description
Zinc bromide (chemical formula: ZnBr2) is an inorganic compound consisting of zinc and bromide. It is manufactured through the reaction between zinc oxide (alternatively, zinc metal) with hydrobromic acid, alternatively by the reaction between zinc metal and bromine. It is a kind of Lewis acid in organic chemistry. It can be used as the electrolyte in the zinc bromide battery. In oil and natural gas industry, its related solution can be used to displace drilling mud. Moreover, its solution can be used as a transparent shield against radiation. Finally, it can be used as a catalyst for the Stereospecific and regioselective reaction between silacyclopropanes with carbonyl compounds.

-
References
https://en.wikipedia.org/wiki/Zinc_bromide
https://www.alfa.com/en/catalog/B22510/ -
Chemical Properties
Zinc bromide is an odourless white crystalline solid or white hygroscopic powder prepared by dissolving zinc carbonate in hydrobromic acid.
Zinc chloride (ZnCl2) is a white granular crystal made by the action of hydrochloric acid on zinc.
Zinc iodide (ZnI2) is a white powder dissolving zinc in ionic acid. Zinc halides are soluble in water, alcohol, and ether. They were all used as halides for the collodion emulsion processes. - Physical properties White crystalline powder; sharp metallic taste; orthorhombic structure; refractive index 1.5452; density 4.20 g/cm3; very hygroscopic; melts at 394°C; vaporizes at 650°C; highly soluble in water 447g/100 mL at 20°C; aqueous solution acidic; very soluble in alcohol, ether, and acetone; soluble in alkali hydroxides and ammonia solution.
- Uses Zinc bromide is an Optimal catalyst for the stereospecific and regioselective reaction of silacyclopropanes with carbonyl compounds. It is also used to make silver bromide collodion emulsions for photography and to shield viewing windows for nuclear reactions.
-
Preparation
Zinc bromide is prepared by mixing barium bromide and zinc sulfate solutions. The product barium sulfate is removed by filtration and the filtrate is evaporated to obtain crystals of zinc bromide: BaBr2 + ZnSO4 → ZnBr2 + BaSO4
Zinc bromide also may be prepared by the action of zinc with hydrobromic acid followed by crystallization. - General Description A white crystalline noncombustible solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Zinc bromide is used in medicine, in photography.
- Air & Water Reactions Hygroscopic. Water soluble
- Reactivity Profile Acidic inorganic salts, such as Zinc bromide, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
- Health Hazard Inhalation of dust may irritate nose and throat. Ingestion can cause irritation or corrosion of the alimentary tract; if large amount is swallowed and not thrown up, drowsiness and other symptoms of bromide poisoning may occur. Contact with eyes or skin causes irritation.
- Flammability and Explosibility Non flammable
-
reaction suitability
reagent type: catalyst
core: zinc - Potential Exposure Zinc bromide is used in photography, rayon manufacturing and medicine
- Shipping UN3260 Corrosive solid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
- Purification Methods Heat ZnBr2 to 300o under vacuum (2x10-2 mm) for 1hour, then sublime it. [Wagenknecht & Juza Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1072 1965.]
- Incompatibilities Keep away from alkali metals. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, metallic sodium, or potassium. Store above 32 ℉/0℃.
Zinc bromide Preparation Products And Raw materials
Raw materials
Preparation Products
Global(459)Suppliers
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-81148696<br/>+86-15536356810
- Email:1022@dideu.com
- Country:China
- ProdList:3882
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
Hebei Jingbo New Material Technology Co., Ltd
- Tel: +8619931165850
- Email:hbjbtech@163.com
- Country:China
- ProdList:1000
- Advantage:58
-
Supplier:
Hebei Saisier Technology Co., LTD
- Tel:+86-18400010335<br/>+86-18034520335
- Email:admin@hbsaisier.cn
- Country:China
- ProdList:1015
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
-
Supplier:
Hebei Longbang Technology Co., LTD
- Tel:+86-18633929156<br/>+86-18633929156
- Email:admin@hblongbang.com
- Country:China
- ProdList:972
- Advantage:58
-
Supplier:
HebeiShuoshengImportandExportco.,Ltd
- Tel:+86-18532138899<br/>+86-18532138899
- Email:L18532138899@163.com
- Country:China
- ProdList:939
- Advantage:58
-
Supplier:
Shanghai Daken Advanced Materials Co.,Ltd
- Tel:+86-371-+86-371-66670886
- Email:info@dakenam.com
- Country:China
- ProdList:19809
- Advantage:58
Related articles
7699-45-8, Zinc bromideRelated Search:
- Phosphorus tribromide Ethidium bromide Zinkamalgam Tetrabutylammonium bromide Benzyl bromide Hydrogen bromide Boron tribromide Clidinium bromide Zinc phosphate Calcium bromide Sodium bromate Ammonium bromide Sodium bromide Cuprous bromide Potassium bromide SILVER BROMIDE TIN (II) BROMIDE Zinc acetate dihydrate
- metal halide
- Zn
- Ultra-High Purity Materials
- Metal and Ceramic Science
- Materials Science
- Crystal Grade Inorganics
- Chemical Synthesis
- Catalysis and Inorganic Chemistry
- Beaded Materials
- Zinc Salts
- 30: Zn
- ZincMetal and Ceramic Science
- Crystal Grade Inorganics
- Chemical Synthesis
- Catalysis and Inorganic Chemistry
- Zinc
- Zinc Salts
- Salts
- Metal and Ceramic Science
- Zn (Zinc) Compounds
- Transition Metal Compounds
- Classes of Metal Compounds
- Inorganics
- 大化工产品
- 其他
- 锌盐
- 其他化工产品
- 其它原料及中间体
- 有机类
- 高端化学
- 精细化工原料
- 锌系化合物
- 无机产品
- 对照品
- 有机产品
- 精细化工品
- 无机化工
- 有机原料
- 原料
- 添加剂
- 化工
- 生化试剂
- 化工原料
- 有机化工原料
- 溴化物
- 无机化工原料
- 有机中间体
- 化工原料-有机化工
- 医药原料
- 无机盐
- 无机化工产品
- Cc溴化合物
- 锌
- 通用试剂
- 催化和无机化学
- 有机化工
- 重金属
- 石油钻井助剂