Zinc bromide: a catalyst in organic synthesis
Introduction
Zinc bromide is a bromide of zinc. It is used in organic chemistry as a Lewis acid, as a transparent shield against radiation, and in the zinc bromide battery. Zinc is a metallic element with the atomic number 30. It is found in nature most often as the mineral sphalerite. Though excess zinc is harmful, it is an essential element for life in smaller amounts, as it is a cofactor for over 300 enzymes and is found in just as many transcription factors.
Ionic compound or covalent
Zinc Bromide is an ionic compound. An ionic compound is formed between oppositely electrostatic charges. The formula for zinc bromide is ZnBr₂. This chemical formula signifies the combination of one zinc ion (Zn²⁺) and two bromide ions (Br⁻). It’s a binary ionic compound where zinc, a metal, forms a 2+ cation, and bromine, a non-metal, forms 1- anions, resulting in a 1:2 ratio.
The catalytic activity in organic reactions
A catalyst is very important in chemistry science, especially in organic synthesis; it can greatly promote chemical processes and reactions, and effectively and sustainably synthesize products. Zinc is a cheap and readily available metal (zinc is easily extracted from minerals in high purity). Low toxicity, cheap and abundance and accessibility of zinc make it probably attractive for application in chemistry catalysis. Among zinc complexes, zinc (II) bromide (ZnBr2) has been used widely as a catalyst in organic synthesis. ZnBr2 is an efficient and popular acid Lewis catalyst among chemists. Recently, the application of ZnBr2 as a catalyst for synthesising organic compounds and heterocyclic molecules has been reported in the literature.
Synthesis of heterocyclic scaffolds
Compounds with a pyrazol ring system have many pharmacological properties and can play important roles in biochemical processes. The synthesis of a library of medicinally valuable 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one derivative via one-pot three-component condensation of various substituted aldehydes, ethyl acetoacetate and phenyl hydrazine/2,4-dinitrophenylhydrazine in water at 60 ℃ under microwave irradiation has developed by Parveen and his coworkers. The template reaction of indole-3-carbaldehyde, phenylhydrazine and ethyl acetoacetate was catalyzed by several metal salts in different reaction mediums to optimize the reaction conditions for synthesising the pyrazol products. 46% of the template product was seen in the absence of a catalyst after 24 h. The maximum yield (98%) was seen using SiO2/ZnBr2 in water at 60 ℃ under microwave irradiation.
Coupling reactions
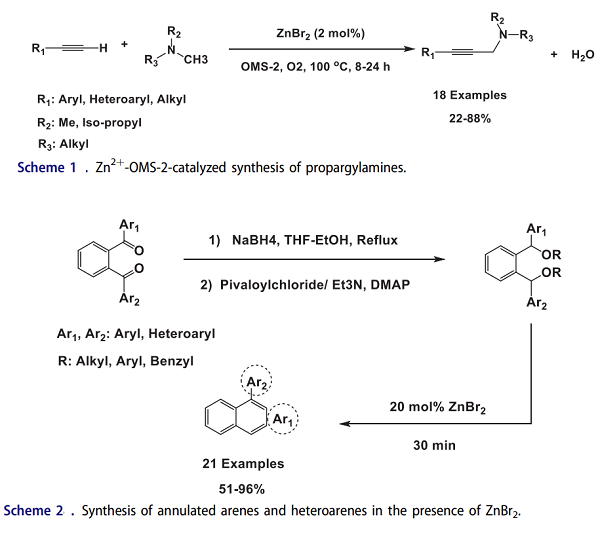
The carbon-carbon and carbon–heteroatom bond formation via coupling reactions is arguably one of the most important transformations in organic synthesis. Mizuno and his coworkers have reported an attractive and efficient approach for the synthesis of propargyl amines from the cross-dehydrogenative coupling of a wide range of terminal alkynes and tertiary amines using molecular oxygen as the terminal oxidant in the presence of ZnBr2 and a manganese oxide-based octahedral molecular sieve (OMS-2) as a highly efficient catalytic system (Scheme 1). The optimized reaction was also studied in the presence of catalytic amounts of ZnF2, ZnCl2, ZnF2, ZnI2, InCl3 and FeCl3, but the observed results were unsatisfactory.
Mohanakrishnan and his coworkers have developed a general and efficient methodology for regioselective annulation of unsymmetrical 1,2-diarylmethinedipivalates in the presence of ZnBr2 as an acid Lewis. The template reaction was performed in the presence of 20 mol % of different Lewis acids/Bronsted acids; the maximum yield (93%) could be obtained using ZnBr2. As seen in Scheme 2, regioselective annulation of unsymmetrical diarylmethine dipivalates using ZnBr2 in DCM at room temperature led to afford a diverse range of annulated arenes and heteroarenes in moderate to high yields. The annulation of the dipivalate proceeds through the intermediacy of benzylic carbocations, followed by intramolecular cyclization and subsequent aromatization to give the annulated products.
Bromination
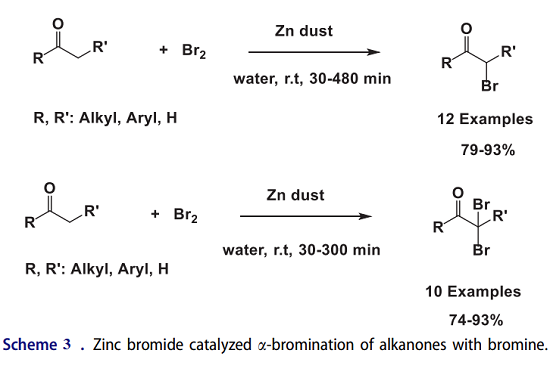
The a-bromination of carbonyl compounds is a hot research topic in organic chemistry, especially in medicinal chemistry. Juneja and his coworkers have utilized zinc bromide as a new and highly active reusable heterogeneous catalyst for the a-bromination of alkanones with bromine in situ–generated zinc bromide from zinc dust and bromine in water (Scheme 3). The template reaction of p-chloroacetophenone with bromine failed in the absence of ZnBr2 in water. To select the best solvent for the a-bromination process, the template reaction was performed in several solvents, and the highest yield was seen in water as solvent.
References:
[1] MASSOUD GHOBADI M K Peiman Pourmoghaddam Qhazvini. Catalytic application of zinc (II) bromide (ZnBr2) in organic synthesis[J]. Synthetic Communications, 2020, 50 24: 3685-3864. DOI:10.1080/00397911.2020.1811873.You may like
See also
Lastest Price from Zinc bromide manufacturers

US $0.10/KG2025-09-14
- CAS:
- 7699-45-8
- Min. Order:
- 1KG
- Purity:
- 99%+
- Supply Ability:
- 2000 kgs

US $6.00/kg2025-04-21
- CAS:
- 7699-45-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month