ChemicalBook > CAS DataBase List > Bestatin
Bestatin
Bestatin
- CAS No.58970-76-6
- Chemical Name:Bestatin
- CBNumber:CB9324019
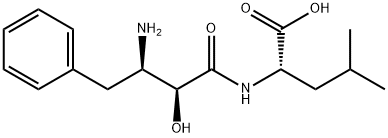
- Molecular Formula:C16H24N2O4
- Formula Weight:308.37
- MOL File:58970-76-6.mol
Bestatin Property
- Melting point 245 °C (dec.)(lit.)
- alpha D20 -15.5° (c = 1.0 in 1N HCl)
- Boiling point 448.76°C (rough estimate)
- Density 1.0917 (rough estimate)
- refractive index 1.5800 (estimate)
- storage temp. Keep in dark place,Sealed in dry,Room Temperature
- solubility Aqueous Acid (Slightly, Sonicated), DMSO (Slightly, Heated)
- pka 8.1, 3.1(at 25℃)
- form Powder
- color White
- optical activity [α]20/D 11°, c = 1 in 1 M NaOH
- Water Solubility Soluble in water (<1 25), DMSO (2 mg/ml), methanol (5 mg/ml), 1eq. NaOH (50 mM), ethanol (<1 mg/ml at 25°C), acetic acid, aq. HCl, and alkaline solution. Insoluble in ethyl acetate, benzene, hexane, and chloroform.
- Merck 13,9910
- InChIKey VGGGPCQERPFHOB-RDBSUJKOSA-N
- CAS DataBase Reference 58970-76-6(CAS DataBase Reference)
- FDA UNII I0J33N5627
- UNSPSC Code 12352209
- NACRES NA.77
Safety
- Safety Statements :22-24/25
- WGK Germany :3
- RTECS :OH2915000
- HS Code :29242998
- Toxicity :LD50 in male, female mice, male, female rats (g/kg): 1.3, 1.9, 1.9, 2.1 s.c.; 0.19, 0.19, 0.90, 0.78 i.p.; >4.0, >4.0, >2.0, >2.0 orally (Sakakibara)
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319-H335
- Precautionary statements P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P362+P364-P403+P233-P501c
Bestatin Price
More Price(13)
- Brand: Sigma-Aldrich
- Product number: 200484-M
- Product name : Bestatin - CAS 58970-76-6 - Calbiochem
- Purity: Binds to cell surfaces and inhibits surface aminopeptidases, notably aminopeptidase B and leucine aminopeptidase.
- Packaging: 200484-10MG
- Price: ₹16670.01
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 200484-M
- Product name : Bestatin - CAS 58970-76-6 - Calbiochem
- Purity: Binds to cell surfaces and inhibits surface aminopeptidases, notably aminopeptidase B and leucine aminopeptidase.
- Packaging: 200484-10MG
- Price: ₹16670.01
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich
- Product number: 200484-M
- Product name : Bestatin - CAS 58970-76-6 - Calbiochem
- Purity: Binds to cell surfaces and inhibits surface aminopeptidases, notably aminopeptidase B and leucine aminopeptidase.
- Packaging: 200484-500MG
- Price: ₹883597.12
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 200484-M
- Product name : Bestatin - CAS 58970-76-6 - Calbiochem
- Purity: Binds to cell surfaces and inhibits surface aminopeptidases, notably aminopeptidase B and leucine aminopeptidase.
- Packaging: 200484-500MG
- Price: ₹883597.12
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich
- Product number: 200484-M
- Product name : Bestatin - CAS 58970-76-6 - Calbiochem
- Purity: Binds to cell surfaces and inhibits surface aminopeptidases, notably aminopeptidase B and leucine aminopeptidase.
- Packaging: 200484-250MG
- Price: ₹1611522.08
- Updated: 2022/06/14
- Buy: Buy
Bestatin Chemical Properties,Usage,Production
- Description Bestatin is an aminopeptidase inhibitor originally isolated from S. olivoreticuli. It inhibits aminopeptidase B (IC50 = 0.05 μg/ml), aminopeptidase N (IC50 = 16.9 μM), leucine aminopeptidase (IC50 = 0.01 μg/ml), and the aminopeptidase activity of leukotriene A4 (LTA4) hydrolase (Kapp = 172 nM). It is selective for these aminopeptidases over aminopeptidase A, trypsin, chymotrypsin, elastase, papain, pepsin, and thermolysin. Bestatin inhibits the production of LTB4 in erythrocytes when used at a concentration of 70 μM. It increases the expression of Akt, inhibits proliferation, migration, and invasion, and induces autophagy and apoptosis in 5637 bladder cancer cells. Bestatin (5 and 15 mg/kg) decreases serum levels of LTB4 and reduces tumor growth in a patient-derived xenograft (PDX) mouse model of colorectal cancer.
- Chemical Properties white powder, soluble in methanol, ethanol, DMSO and other organic solvents, from Streptomyces olivoreticuli.
- Originator Inst. of Microbial Chem (Japan)
- Uses Bestatin is "pseudo"-dipeptide isolated from Strepotmyces olivreticuli in 1976. Bestatin is a potent aminopeptidase B inhibitor with no carboxypeptidase activity. Bestatin acts as an immunomodulator by activating macrophages and T lymphocytes. Bestatin also exhibits good antitumour activity and is has been investigated in clinical trials.
- Uses Ubenimex (also known as bestatin) is a competitive aminopeptidase B inhibitor with an IC50 of 100 mg/ml for K562 cells. Proliferation of all the cell lines except KG1 was inhibited by bestatin. P39/TSU, HL60 and U937 were highly sensitive, with 50% growth inhibitory concentration. It is useful in the treatment of non-lymphocytic leukemia. In other types of cancers ubenimex added to radiotherapy or chemotherapy following surgery significantly inmases survival time through immunopotentiation. It is being studied for use in the treatment of acute myelocytic leukemia.
- Application Bestatin can indirectly act on monocytes by inhibiting Aminopeptidase B, which in turn inhibits the catabolism of tuftsin. Bestatin has also been shown to inhibit Leukotriene A4 hydrolase. It can directly stimulate lymphocytes through its fixation on the cell surface. The agent has been used in multiple leukemia studies. Bestatin is an aminopeptidase inhibitor, inhibits enkephalin metabolism and leukotriene A4 hydrolase. Inhibits tumor cell proliferation.
- brand name Bestatin
- General Description Bestatin was found in the culture broth of Streptomyces olivoreticuli in 1976by Umezawa et al. in the course of screening specific enzyme inhibitors of microbial origin. It shows inhibitory activity against specific exopeptidases, e.g., aminopeptidase B and leucine aminopeptidase. However, it does not act against aminopeptidase A, carboxypeptidases A and B, or endopeptidases. Bestatin acts as a bioresponse modifier and shows antitumor activity via stimulation of immuno response in the host. In combination with cytotoxic anticancer drugs, it is now under clinical evaluation for use in cancer therapy.
- reaction suitability reaction type: solution phase peptide synthesis
- Biological Activity Aminopeptidase inhibitor (K i = 0.001-90 mM); inhibits enkephalin metabolism and leukotriene A 4 hydrolase. Inhibits tumor cell proliferation.
- Safety Profile Poison by intraperitoneal route.Moderately toxic by subcutaneous route. Experimentalreproductive effects. When heated to decomposition itemits toxic fumes of NOx.
- storage Store at +4°C
- Mode of action Bestatin acts as an immunomodulator by activating macrophages and T lymphocytes.
- Clinical claims and research Bestatin (ubenimex) is a potent inhibitor of aminopeptidase N and aminopeptidase B, which was isolated from a culture filtrate of Streptomyces olivoreticuli during the search for specific inhibitors of enzymes present on the membrane of eukaryotic cells. Inhibitors of aminopeptidase activity are associated with macrophage activation and differentiation, and Bestatin has shown significant therapeutic effects in several clinical trials. In one multi-institutional study, patients with acute non-lymphocytic leukemia (ANLL) were randomized to receive either Bestatin or control orally after completion of induction and consolidation therapy, and concomitant with maintenance chemotherapy. Remission duration was prolonged in the Bestatin group, although this difference did not reach statistical significance. However, OS was prolonged in the Bestatin group. Recently, a confirmatory phase III trial in ANLL was reported that extended the observation to a significant prolongation of remission. Bestatin has also shown adjuvant activity when administered to acute leukemia and CML patients who did not develop graft-versus-host disease (GVHD) within 30 days following BMT. Bestatin-treated acute leukemia patients had an increased incidence of chronic low-grade GVHD compared with the control arm and a lower relapse rate. Recently, a phase III study of resected stage 1 squamous cell lung cancer patients treated orally with either Bestatin or placebo daily for 2 years revealed that 5-year cancer-free survival was significantly greater in the Bestatin group (71%) as compared to the placebo group (62%). OS was also significantly improved, as was cancer-free survival. Recent studies in patients with nonsmall cell lung cancer (NSCLCs) suggest that it also has anti-angiogenic activity.
- References 1. umezawa h, aoyagi t, suda h, hamada m, takeuchi t, bestatin, an inhibitor of aminopeptidase b, produced by actinomycetes, j antibiot (tokyo). 1976 jan; 29(1):97-9.2. umezawa, h., aoyagi, t., suda, h., hamada, m., and takeuchi, t. 197615. antibiotics 29, 97.3. suda, h., takita, t., aoyagi, t., and umezawa, h. (1976) j. antibiotics 29, 100.4. nakamura, h., suda, h., takita, t., aoyagi, t., umezawa, h., and iitaka, y. (1976) j. antibiotics 29, 102.5. umezawa, s., tsuchiya, t., and tatsuta, k. (1966) bull. chem. sot. japan 39, 1235. 6. barlow, c. b., and gijthrie, r. d. (1967) j. chem. sot. (c) 1194. 7. bukhari, s. t. k., guthrie, r. d., scott, a. i., and wrixon, a. d. (1970) tetrahedron 26, 3653.8. suda et al. inhibition of aminopeptidase b and leucine aminopeptidase by bestatin and its stereoisomer, archives of biochemistry and biophysics, 77, 196-200 (1976)
Bestatin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(424)Suppliers
-
Supplier:
Chengdu Aslee Biopharmaceuticals, Inc.
- Tel:28-85305008
- Email:
- Country:CHINA
- ProdList:964
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Hubei XinRunde Chemical Co., Ltd.
- Tel:+8615102730682
- Email:bruce@xrdchem.cn
- Country:CHINA
- ProdList:566
- Advantage:55
-
Supplier:
Shanghai Zheyan Biotech Co., Ltd.
- Tel:18017610038
- Email:zheyansh@163.com
- Country:CHINA
- ProdList:3619
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Biochempartner
- Tel:0086-13720134139
- Email:candy@biochempartner.com
- Country:CHINA
- ProdList:965
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Accela ChemBio Inc.
- Tel:+1-858-6993322
- Email:info@accelachem.com
- Country:United States
- ProdList:19825
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
58970-76-6, BestatinRelated Search:
- Tris(hydroxymethyl)aminomethane N-CARBOBENZOXY-DL-LEUCINE ALTRENOGEST 6-Aminocaproic acid mecysteine CHLOROPHOSPHONAZO III Glycine L-tert-Leucine N-(2-HYDROXYETHYL)LACTAMIDE BESTATIN HYDROCHLORIDE H-BETA-ALA-LEU-OH H-BETA-ALA-ALA-OH (-)-N-[(2S, 3R)-3-AMINO-2-HYDROXY-4-PHENYLBUTYRYL]-L-LEUCINE METHYL ESTER THREO-(2RS)-3-ACETYLAMINO-2-HYDROXY-4-OXO-4-PHENYLBUTYRIC ACID [UBENIMEX INTERMEDIATE] Bestatin AMINO ACIDS Ubenimex,AntitumorDrug H-BETA-ALA-DL-LEU-OH
- API
- Bestatin
- peptides
- Active Pharmaceutical Ingredients
- Pepetides
- Ubenimex
- ProteaseInhibitors
- 酶抑制剂
- 中药标准品,对照品
- 抗肿瘤类
- 试剂
- 药靶配体
- 抑制剂
- 化学试剂
- 药品
- 原料
- 生物
- 标准品 -中药标准品
- 对照品-中药对照品
- 蛋白酶抑制剂
- 生化试剂
- 细胞生物学试剂
- 细胞生物学-蛋白提取/检测
- 生化试剂-缓冲剂类
- 医药原料药
- 抗生素类
- 生物化学
- 中药对照品
- 小分子抑制剂
- 植物提取物
- 医药原料
- 抗生素
- 细胞生物学
- 原料药
- 科研试剂
- 杂环类
- 医药中间体
- 标准品
- 微生物代谢物
- 其他科研原料药
- Unnatural Amino Acid Derivatives
- Specialty Synthesis
- Peptide Synthesis
- Leucine Derivatives
- CH32CHCH2CHNHCOCHOHCHNH2CH2C6H5CO2H
- C16H24N2O4
- BESTATIN 贝他定(苯丁抑制素) |CAS 58970-76-6
- 贝他定(氨肽酶抑制剂)
- 抑氨肽酶,乌苯美司,贝他定,苯丁抑制素
- 抑氨肽酶
- 抑氨肽酶,乌苯美司,贝他定
- CD13/APN/LTA4H抑制剂(BESTATIN)
- BESTATIN,AMINOPEPTIDASE抑制剂
- (S)-2-((2S,3R)-3-氨基-2-羟基-4-苯基BUTAN乙酰胺基)-4-甲基戊酸
- 化合物OLMUTINIB
- 乌苯美司/N-[(2S,3R)-3-氨基-2-羟基-4-苯丁酰]-L-亮氨酸/威迪凯/[S-(R*,S*)]-N-(3-氨基-2-羟基-1-氧代-4-苯丁基)-L-亮氨酸/苯丁抑制素
- 乌苯美司对照品
- 贝斯塔丁(UBENIMEX)