ChemicalBook > CAS DataBase List > Bis(2-ethylhexyl) phthalate
Bis(2-ethylhexyl) phthalate
Bis(2-ethylhexyl) phthalate
- CAS No.117-81-7
- Chemical Name:Bis(2-ethylhexyl) phthalate
- CBNumber:CB8708555
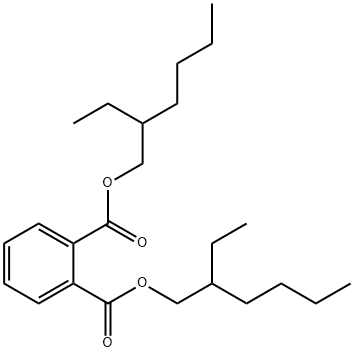
- Molecular Formula:C24H38O4
- Formula Weight:390.56
- MOL File:117-81-7.mol
Bis(2-ethylhexyl) phthalate Property
- Melting point -50 °C
- Boiling point 386 °C (lit.)
- Density 0.985 g/mL at 20 °C (lit.)
- Pour Point -46
- vapor density >16 (vs air)
- vapor pressure 1.2 mm Hg ( 93 °C)
- refractive index n
20/D 1.488 - Flash point 405 °F
- storage temp. 2-8°C
- solubility Miscible with mineral oil and hexane (U.S. EPA, 1985)
- form Liquid
- color APHA: ≤10
- Odor Very slight, characteristic.
- Viscosity 78.17mm2/s
- Water Solubility Negligible
- FreezingPoint <-60℃
- Merck 14,2864
- BRN 1890696
- Henry's Law Constant (x 10-5 atm?m3/mol): 1.1 at 25 °C (calculated, Howard, 1989)
- Exposure limits Potential occupational carcinogen. NIOSH REL: TWA 5, STEL 10, IDLH 5,000; OSHA PEL: TWA 5; ACGIH TLV: TWA 5 (adopted).
- Dielectric constant 5.1(24℃)
- EPA Primary Drinking Water Standard MCL:0.006,MCLG:zero
- LogP 7.5 at 20℃
- Indirect Additives used in Food Contact Substances DI(2-ETHYLHEXYL) PHTHALATE
- FDA 21 CFR 165.118; 175.105; 175.300; 177.1200; 177.1460; 177.2600; 178.3910
- CAS DataBase Reference 117-81-7(CAS DataBase Reference)
- EWG's Food Scores 1
- FDA UNII C42K0PH13C
- Proposition 65 List Di(2-ethylhexyl)phthalate (DEHP)
- IARC 2B (Vol. Sup 7, 77, 101) 2013
- NIST Chemistry Reference Bis(2-ethylhexyl)phthalate(117-81-7)
- EPA Substance Registry System Di(2-ethylhexyl) phthalate (117-81-7)
- UNSPSC Code 41116107
- NACRES NB.61
Safety
- Hazard Codes :T,F
- Risk Statements :60-61-39/23/24/25-23/24/25-11
- Safety Statements :53-45-36/37-16
- RIDADR :UN 1230 3/PG 2
- OEB :B
- OEL :TWA: 5 mg/m3, STEL: 10 mg/m3
- WGK Germany :1
- RTECS :TI0350000
- Autoignition Temperature :734 °F
- TSCA :Yes
- HazardClass :9
- PackingGroup :III
- HS Code :29173400
- Hazardous Substances Data :117-81-7(Hazardous Substances Data)
- Toxicity :Acute oral LD50 for guinea pigs 26 gm/kg, mice 30 gm/kg, rats 30,600 mg/kg, rabbits 34 gm/kg (quoted, RTECS, 1985).
- IDLA :5,000 mg/m3
-
NFPA 704:
1 0
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H360FD
- Precautionary statements P201-P202-P280-P308+P313-P405-P501
Bis(2-ethylhexyl) phthalate Price
More Price(22)
- Brand: Sigma-Aldrich(India)
- Product number: D201154
- Product name : Dioctyl phthalate
- Purity: ≥99.5%
- Packaging: 5ML
- Price: ₹1948.5
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: D201154
- Product name : Dioctyl phthalate
- Purity: ≥99.5%
- Packaging: 500ML
- Price: ₹1948.5
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: D201154
- Product name : Dioctyl phthalate
- Purity: ≥99.5%
- Packaging: 100ML
- Price: ₹1948.5
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: D201154
- Product name : Dioctyl phthalate
- Purity: ≥99.5%
- Packaging: 2L
- Price: ₹5509.93
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: D201154
- Product name : Dioctyl phthalate
- Purity: ≥99.5%
- Packaging: 2.5L
- Price: ₹5953.75
- Updated: 2022/06/14
- Buy: Buy
Bis(2-ethylhexyl) phthalate Chemical Properties,Usage,Production
- Description Di(2-ethylhexyl)phthalate is a colorless oilyliquid with almost no odor. Molecular weight=390.56;Specific gravity (H2O:1)=0.99; Boiling point=385℃;Freezing/Melting point=-50℃; Vapor pressure=,0.01% mmHg at 20℃; Flash point=195℃;Autoignition temperature=348℃. Explosive limits:LEL=0.1%; UEL 2 0.2%. Hazard Identification (based onNFPA-704 M Rating System): Health 1, Flammability 1,Reactivity 0. Slightly soluble in water;solubility=0.00003% at 25℃.
- Chemical Properties Bis(2-ethylhexyl)phthalate is a colorless liquid with a slight odor. It is miscible with mineral oil and hexane, and soluble in most organic solvents. Bis(2-ethylhexyl)phthalate is insoluble in water (NTP, 1994a).
- Uses As plasticizer in PVC applications and flexible vinyls.
- Uses Dialkyl phthalate ester plasticizer used to import softness and flexibility to PVC products.
-
Uses
Bis(2-ethylhexyl) phthalate, known by a variety of other names and usually abbreviated DEHP, was patented in 1950 and first used as vacuum pump oil. But since then, it has become the most widely used plasticizer for poly(vinyl chloride) (PVC) and other plastics. Recently, relatively high levels of DEHP have been found in foods, especially foods high in oils and fats. The U.S. Food and Drug Administration now restricts its use in packaging materials to water-based foods. The European Commission has banned the use of DEHP and other phthalates in PVC toys.
Di(2-ethylhexyl)phthalate (DEHP) is a benzenedicarboxilate ester which is the most widely used plasticizer to soften resins. DEHP is hydrolyzed into what many believe to be its toxic metabolite, mono(2-ethylhexyl)phthalate (MEHP), by lipase enzymes in the gastrointestinal tract. -
Uses
Bis(2-ethylhexyl) phthalate is commonly used plasticizing agent for PVCs, which as such is a component of blood bank bags, surgical tubing, and other products including food wrappers and children’s toys.
- Production Methods DEHP is commercially produced via esterification of phthalic acid anhydride and 2-ethylhexanol. Esterification is completed with an acid or metal catalyst or with no catalyst at high temperature.
- Definition ChEBI: A phthalate ester that is the bis(2-ethylhexyl) ester of benzene-1,2-dicarboxylic acid.
- General Description Colorless to pale yellow oily liquid. Nearly odorless.
- Air & Water Reactions Insoluble in water.
- Reactivity Profile Bis(2-ethylhexyl) phthalate reacts with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides. Incompatible with nitrates .
- Health Hazard Inhalation can cause nausea and irritation of nose and throat. Contact of liquid with eyes or skin causes irritation. Ingestion can cause abdominal cramps, nausea and diarrhea.
-
Health Hazard
The acute oral toxicity is of extremelylow order. The oral LD50 value in miceis in the range 30,000 mg/kg. Ingestion ofabout 10 mL of the liquid may produce gas trointestinal pain, hypermotility, and diar rhea in humans. Chronic administration byoral route did not produce any adverseeffect in animals. A dose of 100 mg/kg/dayproduced increased liver and kidney weight,as well as retardation of growth in dogs.Mice given diets containing 12,000 ppm ofdiethylhexyl phthalate for 40 weeks devel oped renal hyperplasia (Ward et al. 1988).There was no evidence of renal carcino genicity. Oral administration, however, pro duced tumors in livers in the laboratoryanimals. It is a suspected cancer-causingagent, and there is sufficient evidence of itscarcinogenicity in animals. Such evidence inhumans, however, is lacking
Rock and co-workers (1988) reported theinteraction of diethylhexyl phthalate withblood during storage in PVC plastic containers. The plasticizer is leached into theplasma and converted to mono(2-ethylhexyl)phthalate by a plasma enzyme. At a levelof >125 mg/mL in the blood mono(2-ethyl hexyl) phthalate produced a 50% decrease inheart rate and blood pressure in rats. - Flammability and Explosibility Not classified
- Chemical Reactivity Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
- Safety Profile Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Experimental teratogenic data. Other experimental reproductive effects. Poison by intravenous route. Human systemic effects by ingestion: gastrointestinal tract effects. A mild skin and eye irritant. When heated to decomposition it emits acrid smoke.
- Potential Exposure Bis(2-ethylhexyl)phthalate is primarily used as one of several plasticizers in polyvinyl chloride resins used for fabricating flexible vinyl products. It has also been reported as being used as a replacement for polychlorinated biphenyls (PCBs) in dielectric fluids for electric capacitors and in vacuum pumps (NTP, 1994a; Merck, 1989). The primary stationary sources that have reported emissions of bis(2-ethylhexyl)phthalate in California are furniture and fixtures manufacturing, and lumber and wood products manufacturing (ARB, 1997b).
- First aid If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
- Carcinogenicity Di(2-ethylhexyl) phthalate (DEHP) is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
-
Source
MCLG: zero; MCL: 6 μg/L. In addition, a DWEL of 700 μg/L
was recommended (U.S. EPA, 2000).
Detected in distilled water-soluble fractions of new and used motor oil at concentrations of 17–21 and ND-1.2 μg/L, respectively (Chen et al., 1994). Also may leach from plastic products used in analytical laboratories (e.g., tubing, containers). -
Environmental Fate
Biological. Bis(2-ethylhexyl) phthalate degraded in both amended and unamended calcareous
soils from New Mexico. After 146 d, 76 to 93% degraded (mineralized) to carbon dioxide. No
other metabolites were detected (Fairbanks et al., 1985). In a 56-d experiment, [14C]bis(2-
ethylhexyl) phthalate applied to soil-water suspensions under aerobic and anaerobic conditions
gave 14CO2 yields of 11.6 and 8.1%, respectively (Scheunert et al., 1987).
When bis(2-ethylhexyl) phthalate was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum, no degradation was observed after 7 d. In a 21-d period, however, gradual adaptation did occur resulting in 95 and 93% losses at concentrations of 5 and 10 mg/L, respectively (Tabak et al., 1981).
Half-lives of 19 h and 4–5 wk were reported for bis(2-ethylhexyl) phthalate in activated sludge and river water, respectively (Saeger and Tucker, 1976). In sludge-amended agricultural soil, bis(2-ethylhexyl) phthalate (initial concentration 4.1 nmol/g) degraded at a rate of 25.6 pmol/g·day via the intermediate o-phthalic acid. Despite the presence of microorganisms capable of degrading bis(2-ethylhexyl) phthalate, >40% of the bis(2-ethylhexyl) phthalate added was not mineralized after 1 yr (Roslev et al., 1998). Aerobic degradation of bis(2-ethylhexyl) phthalate by acclimated soil and activated sewage sludge microbes was studied using an acclimated shake flask CO2 evolution test. After 28 d, loss of bis(2-ethylhexyl) phthalate (primary degradation) was >99%, with a lag phase of 2.7 d, and ultimate biodegradation (CO2 evolution) was 86%. The half-life under these conditions was 4.55 d (Sugatt et al., 1984).
In the presence of suspended natural populations from unpolluted aquatic systems, the secondorder microbial transformation rate constant determined in the laboratory was reported to be 4.2 ± 0.7 x 10-15 L/organism?h (Steen, 1991). Using the modified Sturm test, >80% of bis(2-ethylhexyl) phthalate mineralized to carbon dioxide in 28 d (Scholz et al., 1997).
Surface Water. In laboratory experiments, the extrapolated volatilization half-life of bis(2- ethylhexyl) phthalate in a stirred water vessel (outer dimensions 22 x 10 x 21 cm) conducted at 22 and 30 °C and an air flow rate of 0.20 m/sec is 3,500 h (Kl?pffer et al., 1982).
Photolytic. The estimated photolytic half-life of bis(2-ethylhexyl) phthalate in water is 143 d (Wolfe et al., 1980).
Chemical/Physical. Hydrolyzes in water to o-phthalic acid (via the intermediate 2-ethyl-hexyl hydrogen phthalate) and 2-ethylhexyl alcohol (Kollig, 1993; Wolfe et al., 1980). Although no pH value was given, the reported hydrolysis rate constant under alkaline conditions is 1,400/M?yr (Ellington et al., 1993; Kollig, 1993). A second-order rate constant of 1.1 x 10-4/M?sec was reported for the hydrolysis bis(2-ethylhexyl) phthalate at 30 °C and pH 8 (Wolfe et al., 1980). Kl?pffer et al. (1982) estimated an evaporation half-life of approximately 140 d from a 21-cm deep vessel. - Metabolism Bis(2-ethylhexyl) phthalate is metabolized in fish by enzymatic hydrolysis to mono-2-ethylhexyl phthalate, phthalic acid, and glucuronides of these compounds (Stalling et al., 1973).
- storage Color Code—Green: General storage may be used.Prior to working with DEHP you should be trained on itsproper handling and storage. Before entering confined spacewhere this chemical may be present, check to make surethat an explosive concentration does not exist. Bis (2-ethylhexyl)phthalate must be stored to avoid contact with oxidizing materials, such as permanganates, nitrates, peroxides,chlorates, and perchlorates, since violent reactions occur.Store in tightly closed containers in a cool, well-ventilatedarea away from heat. Sources of ignition, such as smokingand open flames, are prohibited where bis (2-ethylhexyl)phthalate is used, handled, or stored in a manner that couldcreate a potential fire or explosion hazard. Sources of ignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard. Metalcontainers involving the transfer of=gallons or more ofthis chemical should be grounded and bonded. Drums mustbe equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof this chemical. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
- Shipping The name of this material is not on the DOT listof materials for label and packaging standards. However,based on regulations, it may be classified as anEnvironmentally hazardous substances, liquid, n.o.s. It fallsin Hazard Class 9 and Packing Group III.[20,21]
- Purification Methods Wash the ester with Na2CO3 solution, then shake it with water. After the resulting emulsion has been broken by adding ether, the ethereal solution is washed twice with water, dried (CaCl2), and evaporated. The residual liquid is distilled several times under reduced pressure, then stored in a vacuum desiccator over P2O5 [French & Singer J Chem Soc 1424 1956]. [Beilstein 9 IV 3184.]
- Incompatibilities Nitrates, strong oxidizers, strong acids,strong alkalis.
- Toxics Screening Level There was previously no Initial Threshold Screening Level (ITSL) for di-(2-ethylhexyl) phthalate (DEHP). The ITSL is now being established at 70 μg/m3 based on an annual averaging time.
Bis(2-ethylhexyl) phthalate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(765)Suppliers
- Americas 1 Argentina 1 Australia 4 Belgium 4 Brazil 3 Canada 12 Chile 1 China 410 Costa Rica 1 Czech 1 Czech Republic 3 Europe 5 France 3 Germany 15 Hungary 1 India 122 Indonesia 2 Iran 1 Italy 3 Japan 13 Jordan 2 Lebanon 1 Malaysia 3 Mexico 4 Norway 1 Philippines 1 Russia 2 Saudi Arabia 1 Singapore 2 Slovakia 1 South Korea 11 Spain 1 Sweden 1 Switzerland 2 Thailand 1 The Netherlands 7 Ukraine 1 United Arab Emirates 4 United Kingdom 19 United States 94 Global 765
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12814
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5870
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
Yujiang Chemical (Shandong) Co.,Ltd.
- Tel: +8617736087130
- Email:catherine@yjchem.com.cn
- Country:China
- ProdList:994
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
-
Supplier:
Hebei Andu Technology Com.,Ltd
- Tel:+86-17798073498<br/>+86-17798073498
- Email:admin@hbandu.com
- Country:China
- ProdList:378
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21629
- Advantage:55
Related articles
117-81-7, Bis(2-ethylhexyl) phthalateRelated Search:
- DI-N-OCTYL PHTHALATE N-Phthaloylglycine Dibutyl phthalate Diethyl phthalate Dimethyl phthalate Diallyl phthalate Potassium hydrogen phthalate DI-N-HEXYL PHTHALATE Benzyl butyl phthalate DIPHENYL PHTHALATE Phthalhydrazide DI-N-PENTYL PHTHALATE-D4 Dinonyl phthalate Diisononyl phthalate Dicyclohexyl phthalate PHTHALIC ACID DISODIUM SALT Diisodecyl phthalate Didecyl phthalate
- 117-81-7
- ester series
- Aromatics
- Volatiles/ Semivolatiles
- Oeko-Tex Standard 100
- European Community: ISO and DIN
- B
- Alpha Sort
- Allergens
- A-BAlphabetic
- 2000/60/EC
- Plasticizers (phthalates)Method Specific
- PhthalatesMethod Specific
- Plasticizer
- Phthalates (Plasticizer)
- Phthalates (Environmental Endocrine Disruptors)
- Functional Materials
- Environmental Endocrine Disruptors
- Analytical Chemistry
- Piperazine derivates
- BI - BZCosmetics
- Phthalates
- Food additive
- 中药标准品,对照品
- 抑制剂
- 其它原料及中间体
- 标准品-增塑剂
- 国家标准物质
- 高端化学
- 有机溶剂
- 有机类
- 国标产品
- 大化工产品
- 液体类
- 原料药
- 有机产品
- 化学试剂
- 有机原料
- 中药对照品
- 化工
- 通用生化试剂-有机酸
- 有机中间体
- 化工材料
- 精细化工原料
- 有机羧酸及其衍生物
- 液体化工
- 有机化工原料
- 增塑剂
- 能源类
- 化工中间体工业原料
- 其它酚类
- 标准品 -对照药材
- 有机化工原料
- 助剂
- 化工原料
- 主要产品
- 有机酯类
- 乳化剂