ChemicalBook > CAS DataBase List > (S)-(+)-Ketoprofen
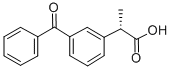
(S)-(+)-Ketoprofen
(S)-(+)-Ketoprofen
- CAS No.22161-81-5
- Chemical Name:(S)-(+)-Ketoprofen
- CBNumber:CB8356545
- Molecular Formula:C16H14O3
- Formula Weight:254.28
- MOL File:22161-81-5.mol
(S)-(+)-Ketoprofen Property
- Melting point 75-78 °C(lit.)
- Boiling point 431.3±28.0 °C(Predicted)
- Density 1.198±0.06 g/cm3(Predicted)
- storage temp. Sealed in dry,Room Temperature
- solubility insoluble in H2O; ≥10.6 mg/mL in DMSO; ≥20.55 mg/mL in EtOH
- pka 4.23±0.10(Predicted)
- form White solid.
- color White to off-white
- optical activity [α]22/D +49°, c = 1 in methanol
- CAS DataBase Reference 22161-81-5(CAS DataBase Reference)
- FDA UNII 6KD9E78X68
- ATC code M01AE17,M02AA27
- UNSPSC Code 12352115
- NACRES NA.22
Safety
- Hazard Codes :Xn,T
- Risk Statements :22-36/37/38-50/53-25
- Safety Statements :26-60-61-45
- RIDADR :UN 2811 6.1/PG 3
- WGK Germany :3
- RTECS :CY1572790
-
NFPA 704:
0 4 0
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H301-H315-H319-H335-H400
- Precautionary statements P273-P301+P310+P330-P302+P352-P305+P351+P338
(S)-(+)-Ketoprofen Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 471909
- Product name : (S)-(+)-Ketoprofen
- Purity: 99%
- Packaging: 1G
- Price: ₹10900.78
- Updated: 2022/06/14
- Buy: Buy
(S)-(+)-Ketoprofen Chemical Properties,Usage,Production
- Chemical Properties White Solid
- Uses Anti-inflammatory; analgesic
- Uses COX inhibitor
- Definition ChEBI: A monocarboxylic acid that is (S)-hydratropic acid substituted at position 3 on the phenyl ring by a benzoyl group. A cyclooxygenase inhibitor, it is used to relieve short-term pain, such as muscular pain, dental pain and dysmenorrhoea.
- Biological Activity (s)-ketoprofen, a dual cox1/2 inhibitor, can be used as a nonsteroidal anti-inflammatory drug to treat arthritis-related inflammatory pains. ketoprofen is photolabile and undergoes degradation when irradiated by sunlight to induce various skin diseases [1].
- in vitro the combination of uvb irradiation with ketoprofen dose-dependently induced the cytotoxicity and suppressed dna synthesis in hacat cells. uvb-irradiated kp inhibited the cell growth and induced g2/m cell cycle arrest by regulating the levels of cdc2, cyclin b1, chk1, tyr15-phosphorylated cdc2 and p21. the dapi staining results has revealed that kp accentuated the apoptotic response to uvb radiation in hacat cells [1].
- in vivo in a placebo-controlled, double-blind study in the rhesus monkeys macaca mulatta with periodontal disease, administeration of kp at 1% level in suitable topical vehicles to the gingiva once daily at a standard dose of 1.8 ml per monkey for 6 months effectively inhibited gcf-ltb4 and gcf-pge2 and positively altered alveolar bone activity [2]. ketoprofen at a dose of 3.63 mg/kg bwt (phenylbutazone equimolar dose) showed significant analgesic effects and reduced hoof pain and lameness to a greater extent [3]. treatment with ketoprofen (40 and 80 mg/kg diet) greatly reduced the incidence of transitional cell carcinoma of the urinary bladder by >70% from that seen in dietary mice [4].
-
Drug interactions
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists: antagonism of hypotensive effect; increased risk of nephrotoxicity and hyperkalaemia
Analgesics: avoid concomitant use of 2 or more NSAIDs, including aspirin (increased side effects);avoid with ketorolac (increased risk of side effects and haemorrhage).
Antibacterials: possibly increased risk of convulsions with quinolones
Anticoagulants: effects of coumarins and phenindione enhanced; possibly increased risk of bleeding with heparins, dabigatran and edoxaban - avoid long term use with edoxaban
Antidepressants: increased risk of bleeding with SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas enhanced.
Antiepileptics: possibly increased phenytoin concentration.
Antivirals: increased risk of haematological toxicity with zidovudine; concentration possibly increased by ritonavir.
Ciclosporin: may potentiate nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate; increased risk of bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity; antagonism of diuretic effect, hyperkalaemia with potassium-sparing diuretics.
Lithium: excretion decreased.
Pentoxifylline: increased risk of bleeding
Probenecid: excretion reduced by probenecid.
Tacrolimus: increased risk of nephrotoxicity - Metabolism Dexketoprofen is the S-enantiomer of ketoprofen.The main elimination route for dexketoprofen is glucuronide conjugation in the liver followed by renal excretion.
-
References
[1]. liu s, mizu h, yamauchi h. molecular response to phototoxic stress of uvb-irradiated ketoprofen through arresting cell cycle in g2/m phase and inducing apoptosis[j]. biochemical and biophysical research communications, 2007, 364(3): 650-655.
[2]. li k l, vogel r, jeffcoat m k, et al. the effect of ketoprofen creams on periodontal disease in rhesus monkeys[j]. journal of periodontal research, 1996, 31(8): 525-532.
[3]. owens j g, kamerling s g, stanton s r, et al. effects of ketoprofen and phenylbutazone on chronic hoof pain and lameness in the horse[j]. equine veterinary journal, 1995, 27(4): 296-300.
[4]. hawk e t, kelloff g j, mccormick d l. differential activity of aspirin, ketoprofen and sulindac as cancer chemopreventive agents in the mouse urinary bladder[j]. carcinogenesis, 1996, 17(5): 1435-1438.
(S)-(+)-Ketoprofen Preparation Products And Raw materials
Raw materials
Preparation Products
Global(264)Suppliers
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co Ltd
- Tel:+86-16264648883<br/>+86-16264648883
- Email:niki@zlchemi.com
- Country:China
- ProdList:7245
- Advantage:58
-
Supplier:
Shandong Xuhuang New material CoLTD
- Tel:+86-15095020839<br/>+86-17660422208
- Email:
- Country:China
- ProdList:726
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
SHANDONG ZHI SHANG CHEMICAL CO.LTD
- Tel: +86 18953170293
- Email:sales@sdzschem.com
- Country:China
- ProdList:2930
- Advantage:58
-
Supplier:
Nanjing Dolon Biotechnology Co.,Ltd.
- Tel:18905173768
- Email:sales@dolonchem.com
- Country:CHINA
- ProdList:2972
- Advantage:58
-
Supplier:
Standardpharm Co. Ltd.
- Tel:86-714-3992388
- Email:overseasales1@yongstandards.com
- Country:United States
- ProdList:14332
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
22161-81-5, (S)-(+)-KetoprofenRelated Search:
- Imidocarb dipropionate (RS)-Ketoprofen,KETOPROFEN EPK(CRM STANDARD),KETOPROFEN, USP23,KETOPROFEN(RG),KETOPROFEN(2-(3-BENZOYLPHENYL)PROPIONICACID),Racemic ketoprofen,KETOPROFEN,BP Folic acid Ethyl 2-(Chlorosulfonyl)acetate Nandrolone phenylpropionate 3-(4-Hydroxyphenyl)propionic acid (S)-(+)-2-Phenylpropionic acid Citric acid L-Ketoprofen trometamol Propionic anhydride Propionic acid Desmethyl Ketoprofen rac-4'-Methyl Ketoprofen Allethrin Ascoric Acid KETOPROFEN TROMETHAMINE AMIDE 3-(CYANOMETHYL)BENZOIC ACID 3-ACETYLBENZOPHENONE
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Chiral Compound
- Chiral Reagents
- Aromatics
- Other APIs
- 杂质对照品
- 抑制剂
- 高端化学
- 兽用原料
- 化学试剂
- 原料药
- 日用化学品
- 化学试剂-科研试剂
- 化学原料
- 系列1
- 原料
- 化合物:原料药
- 医药原料
- 小分子抑制剂
- 化学连接
- Chiral Building Blocks
- Cell Signaling Enzymes
- Carboxylic Acids
- Application Index
- Asymmetric Synthesis
- BioChemical
- Biochemicals and Reagents
- Organic Building Blocks
- Cyclooxygenase (COX) Enzymes
- Cyclooxygenase Inhibitors
- Enzymes, Inhibitors, and Substrates
- C6H5COC6H4CHCH3COOH
- C6H5COC6H4CHCH3CO2H
- (S)-(+)-酮洛芬,10 MM DMSO 溶液
- 酮基布洛芬杂质13(S异构体)
- (S)-酮洛芬(酮洛芬杂质3)
- 右酮洛芬杂质8
- 酮洛芬18
- (S)-(+)-酮洛芬/右旋酮洛芬
- 酮洛芬系列/右旋酮洛芬
- 右旋酮洛芬DEXKETOPROFEN
- 酮洛芬杂质10
- Α-甲基-3-苯甲酰基苯乙酸
- 右旋酮洛芬原料
- (S)-2-(3-苯甲酰苯基)异丙酸
- (S)-(+)-2-(3-苯甲酰基苯基)丙酸
- 右旋酮洛芬
- 酮基布洛芬杂质
- 酮洛芬S-异构体
- (S)-(+)-3-苯甲酰基-Α-甲基苯乙酸
- 右酮洛芬
- (S)-(+)-酮洛芬
- 22161-81-5
- (S)-(+)-Ketoprofen - Bio-X ?
- (<i>S</i>)-(+)-Ketoprofen
- S-(+)-Ketoprofen, 10 mM in DMSO
- levoketoprofen