ChemicalBook > CAS DataBase List > ALOSETRON
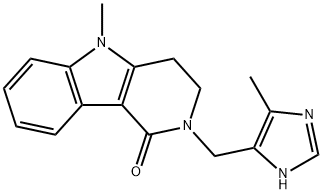
ALOSETRON
ALOSETRON
- CAS No.122852-42-0
- Chemical Name:ALOSETRON
- CBNumber:CB8149787
- Molecular Formula:C17H18N4O
- Formula Weight:294.35
- MOL File:122852-42-0.mol
ALOSETRON Property
- Melting point 238-240 °C
- Boiling point 648.1±55.0 °C(Predicted)
- Density 1.34±0.1 g/cm3(Predicted)
- storage temp. Store at -20°C
- solubility DMSO
- form Powder
- pka 14.10±0.10(Predicted)
- FDA UNII 13Z9HTH115
- ATC code A03AE01
Safety
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
ALOSETRON Chemical Properties,Usage,Production
- Chemical Properties crystalline powder
- Originator Alosetron hydrochloride,GlaxoSmithKline
- Uses Anti-emetic.
- Definition ChEBI: A pyrido[4,3-b]indole compound having a 5-methyl-1H-imidazol-4-ylmethyl group at the 2-position.
- Indications Alosetron (Lotronex) is a 5-HT3 receptor antagonist. Blocking this receptor results in decreased GI motility. Alosetron received FDA approval in February 2000 for the treatment of women with diarrhea-predominant IBS. In November 2000, at the request of the FDA, the drug was voluntarily withdrawn due to reported cases of ischemic colitis, including some fatalities.
-
Manufacturing Process
4-(Chloromethyl)-1-(triphenylmethyl)-1H-imidazole.
Thionyl chloride (0.829 g) was added over 1 min to a stirred suspension of 1- (triphenylmethyl)-1H-imidazole-4-methanol (1.3 g) in a mixture of dichloromethane (50 ml) and DMF (1.0 ml) at 23°C. The solution so obtained was stirred for 15 min. and extracted with 8% sodium bicarbonate solution (80 ml). The organic phase was washed with water (50 ml), dried and evaporated to give an oil which solidified. The solid was slurried in hexane and filtered to give the title compound (1.28 g), m.p. 139-141°C.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one oxime.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one (1.7 g) and hydroxylamine hydrochloride (1.925 g) in pyridine were heated at 60°C for 18 h and cooled. The reaction mixture was evaporated in vacuo to a residue to which was added 8% sodium bicarbonate (150 ml). Extraction with ethyl acetate (300 ml) produced a suspension in the organic layer; this layer and associated solid was separated from the aqueous layer. The aqueous layer was re-extracted with ethyl acetate (250 ml). The combined organic extracts (and suspended solid) were evaporated to a residue, boiled with a mixture of ethanol (150 ml) and methanol (150 ml) and cooled to 50°C. The residue was adsorbed from this solution on to FCC silica and applied to an FCC column. Elution with ethyl acetate/3-10% methanol provided the title compound (1.69 g), m.p. 219- 224°C (decomp.).
2,3,4,5-Tetrahydro-5-methyl-1H-pyrido[4,3-b]indol-1-one.
3,4-Dihydro-4-methylcyclopent[b]indol-1(2H)-one oxime (1.53 g), polyphosphoric acid (409 g) and dioxan (15 ml) were heated at 110-120°C for 2.2 h under nitrogen. The reaction mixture was cooled, and treated with 2 N sodium carbonate solution (1 L). The suspension was extracted with ethyl acetate (4x400 ml) and the combined extracts were dried. Evaporation gave a solid (1.43 g) which was recrystallised from ethyl acetate/cyclohexane. This solid was purified by FCC, eluting with dichloromethane:ethanol:ammonia solution (200:10:1) to give a solid (1.26 g) which was recrystallised from ethanol to provide the title compound (960 mg), m.p. 234-238°C.
2,3,4,5-Tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-1Hpyrido[ 4,3-b]indol-1-one maleate.
A mixture of 2,3,4,5-tetrahydro-5-methyl-1H-pyrido[4,3-b]indol-1-one (0.6 g) and 78% sodium hydride dispersion in mineral oil (0.109 g) in dry DMF (15 ml) was stirred under nitrogen at 50°C until hydrogen evolution ceased (ca. 1.5 h). The mixture was cooled to 40°C and a solution of 4-(chloromethyl)-5- methyl-1-(triphenylmethyl)-1H-imidazole (1.12 g) in dry THF (15 ml) was added. The reaction was then stirred at 40°C for 3 h, at 20°C for 16 h and a further portion of 4-(chloromethyl)-5-methyl-1-(triphenylmethyl)-1H-imidazole (1.12 g) in dry THF (15 ml) was added. The resulting mixture was heated at 40°C for 3 h, quenched with water (20 ml) and acetic acid (20 ml), and heated at 100°C for 2 h. The mixture was then concentrated in vacuo to ca. 60 ml, diluted with 1 M hydrochloric acid (40 ml) and washed with ethyl acetate (3x50 ml). The organic phase was discarded and the acidic aqueous phase was basified (pH=9) with potassium carbonate and extracted with ethyl acetate:ethanol (20:1; 3x100 ml). The extracts were combined, dried and evaporated to give a brown gum (ca. 1 g). This gum was adsorbed onto silica and purified by FCC eluting with dichloromethane:ethanol:ammonia solition (100:8:1) to give a pale brown solid (0.8 g); m.p. 238-240°C (decomp.). This solid was dissolved in a mixture of (hot ethanol and methanol (1:1; 100 ml) and treated with an ethanolic solution of maleic acid (3.18 g). The resulting solution was concentrated to ca. 20 ml and diluted with dry diethyl ether (ca. 8 ml) to precipitate the title compound (0.75 g) as an off-white solid melting point 160-162°C. Hydrochloride may be prepared by treating the above solid with an equivalent of an ethanolic solution of HCl. - brand name Lotronex (GlaxoSmithKline).
- Therapeutic Function Antidiarrheal
ALOSETRON Preparation Products And Raw materials
Raw materials
Preparation Products
Global(91)Suppliers
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
HANGZHOU CLAP TECHNOLOGY CO.,LTD
- Tel:86-571-88216897,88216896<br/>13588875226
- Email:sales@hzclap.com
- Country:CHINA
- ProdList:6312
- Advantage:58
-
Supplier:
Xi'an MC Biotech, Co., Ltd.
- Tel:029-89275612<br/>+8618991951683
- Email:mcbio_sales@163.com
- Country:China
- ProdList:2251
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
-
Supplier:
Baoji Guokang Bio-Technology Co., Ltd.
- Tel:0917-3909592<br/>13892490616
- Email:gksales1@gk-bio.com
- Country:China
- ProdList:9304
- Advantage:58
-
Supplier:
Sinoway Industrial co., ltd.
- Tel:0592-5800732;<br/>+8613806035118
- Email:xie@china-sinoway.com
- Country:China
- ProdList:987
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418684<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:25356
- Advantage:58
-
Supplier:
Hangzhou MolCore BioPharmatech Co.,Ltd.
- Tel:+86-057181025280;<br/>+8617767106207
- Email:sales@molcore.com
- Country:China
- ProdList:49734
- Advantage:58
122852-42-0, ALOSETRONRelated Search:
- Phenylbutazone IMIPRAMINE Cytarabine Chlorpromazine ONDANSETRON FOR LC SYSTEM SUITABILITY Thalidomide ALOSETRON Lurosetron ALOSETRON HCL,ALOSETRON HYDROCHLORIDE 2,3,4,5-Tetrahydro-5-methyl-1H-pyrido[4,3-b]indol-1-one 2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole 3-CARBAMOYL-2-METHYLINDOLE Alosetron-d3 Hydrochloride (1-Methyl-1H-indol-3-yl)-methylamine 1H-Indole-3-carboxamide(9CI) 1-METHYL-4,5,6,7-TETRAHYDRO-1H-PYRROLO[3,2-C]PYRIDINE 2-METHYL-3-N,N-DIMETHYLAMINOMETHYLINDOLE N-(2-(1-METHYL-1H-PYRROL-2-YL)ETHYL)FORMAMIDE
- 有机中间体
- 阿洛司琼-13CD3标准物质
- 9-甲基-3-(5-甲基-3H-咪唑-4-基甲基)-1,2,3,9-四氢-咔唑-4-一盐酸盐
- 阿洛司琼
- 122852-42-0
- aloosetron.
- 9-methyl-3-(5-methyl-3H-Imidazol-4-ylmethyl)-1,2,3,9-Tetrahydro-Carbazol-4-One Hydrochloride
- 9-methyl-3-(5-methyl-3H-Imidazol-4-ylmethyl)- 1,2,3,9-Tetrahydro-
- ALOSETRON USP/EP/BP
- 1H-Pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(4-methyl-1H-imidazol-5-yl)methyl]-
- GR 68755; GR 68755X; LOTRONEX;GR-68755;GR68755
- Alosetron (GR-68755X
- GR-68755X;GR-68755
- 5-METHYL-2-[(5-METHYL-1H-IMIDAZOL-4-YL)METHYL]-3,4-DIHYDROPYRIDO[4,3-B]INDOL-1-ONE
- 5-Methyl-2-[(4-methyl-1H-imidazol-5-yl)methyl]-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one
- ALOSETRON 2,3,4,5-Tetrahydro-5-methyl-2-((5-methyl-1H-imidazol-4-yl)methyl)-1H-pyrido(4,3-b)indol-1-one
- ALOSETRON