Uses
An intermediate in the preparation of HIV-1 specific reverse transcriptase inhibitors, antiallergic agents, and Omeprazole metabolites.
Synthesis
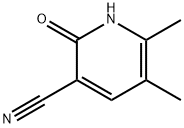
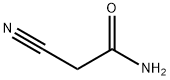
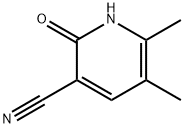
The general procedure for the synthesis of 2-hydroxy-3-cyano-5,6-dimethylpyridine from the compound (CAS:143193-92-4) and cyanoacetamide was as follows: sodium (E/Z)-2-methyl-3-oxo-1-en-1-ol (77.6 g, 0.64 mol) was added to 480 mL of water, followed by the addition of cyanoacetamide (49.0 g, 0.70 mol). To the mixture was added a piperidine-acetic acid solution prepared from acetic acid (9.16 g, 8.75 mL, 0.15 mol), 21.2 mL of water and pyridine (13.0 g, 15.1 mL, 0.15 mol). The mixture was heated at reflux for 4 hours and then stirred at room temperature for 16 hours. Upon completion of the reaction, acetic acid (75.5 g, 72 mL, 1.26 mol) was added to the reaction mixture, prompting the precipitation of a pale yellow solid. The precipitate was collected by diafiltration, washed with water and dried under reduced pressure at 55 °C for 2 hours. A final 66.4 g (70% of theoretical yield) of light yellow solid product was obtained.
References
[1] Patent: CN105530814, 2016, A. Location in patent: Paragraph 0328; 0329; 0330
[2] Journal of Heterocyclic Chemistry, 1987, vol. 24, p. 351 - 355