ChemicalBook > CAS DataBase List > Vildagliptin
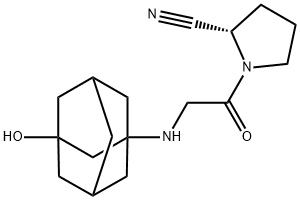
Vildagliptin
Vildagliptin
- CAS No.274901-16-5
- Chemical Name:Vildagliptin
- CBNumber:CB71449010
- Molecular Formula:C17H25N3O2
- Formula Weight:303.4
- MOL File:274901-16-5.mol
Vildagliptin Property
- Melting point 153-155?C
- alpha -78.3° (c = 9.73 in methanol)
- Boiling point 531.3±50.0 °C(Predicted)
- Density 1.27
- Flash point 275.1℃
- storage temp. Keep in dark place,Inert atmosphere,Store in freezer, under -20°C
- solubility Soluble in DMSO (up to 45 mg/ml), in DMF (up to 20 mg/ml), or in Ethanol (up to 20 mg/ml).
- form solid
- pka 15.05±0.40(Predicted)
- color White
- optical activity [α]/D -81 to -91°, c =0.1 in methanol
- Stability Stable for 2 years from date of purchase as supplied. Solutions in DMSO, DMF, or ethanol may be stored at -20°C for up to 3 months
- InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1
- InChIKey SYOKIDBDQMKNDQ-XWTIBIIYSA-N
- SMILES N1(C(CNC23CC4CC(CC(O)(C4)C2)C3)=O)CCC[C@H]1C#N
- CAS DataBase Reference 274901-16-5(CAS DataBase Reference)
- FDA UNII I6B4B2U96P
- NCI Drug Dictionary vildagliptin
- ATC code A10BH02
- UNSPSC Code 12352200
- NACRES NA.77
Safety
- Risk Statements :28-38-41-48
- Safety Statements :24/25-26-28-36/37/39
- RIDADR :3077
- HS Code :29339900
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H332-H335
- Precautionary statements P261-P280-P305+P351+P338
Vildagliptin Chemical Properties,Usage,Production
- Uses Glyptins are class of oral anti-hyperglycemic agents that inhibit dipeptidyl peptidase 4 (DPP-4), which can act as a serine exopeptidase. Aside from their use in type 2 diabetes, gliptins have positive cardiovascular and anti-inflammtatory effects. Antidiabetic.
-
Indications and Usage
Vildagliptin (vildagliptin), developed by Novartis (Novartis) Pharmaceutical Co., Ltd, is another oral administrated inhibitor of Dipeptidyl peptidase-IV after sitagliptin (sitagliptin). In 2008, it is approved for marketing in the European Union for the treatment of type 2 diabetes.
Diabetes is a chronic metabolic disease with its prevalence being increased year by year. Type 2 diabetes is a complicated diseases caused with the combined action between polygenic genetic factors and environmental factors.
Dipeptidyl peptidase IV (DPP-IV) inhibitors are a new class of anti-diabetic drugs which induces and facilitate the biosynthesis and secretion of insulin. It plays a role in lowering the blood carbohydrate concentration through multiple mechanisms such as inhibiting the β cell apoptosis, inhibiting glucagon secretion and reducing food intake. During its lowering effect on reducing blood carbohydrate levels, it can also reverse the situation of deteriorating function of pancreas islet for diabetic patients at the same time. Vildagliptin is the representative of drugs among the dipeptidyl peptidase inhibitor. It exhibited a good anti-diabetic effects and tolerance no matter whether it is being administered alone or in combination with metformin and insulin medication in clinical studies. - Mechanisms of Action Vildagliptin is a selective, competitive and reversible DPP-4 inhibitor. Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 21 (GLP21) are important hormones that regulate body glucose concentration and have the same functions as intestinal insulin. Type-2 diabetes patients suffer from GIP insulin secretion failure, so only their GLP21 can promote insulin secretion by affecting receptors on islet beta cell membranes. GLP21 can also inhibit glucagon secretion and inhibit gastric clearing to extend the feeling of fullness (control appetite). DPP-4 binds with proteins in many types of tissues, including tissue in the kidney, liver, small intestine brush border, pancreatic duct, lymph cells, and endothelial cells, and it can deactivate GLP21 by hydrolyzing its N-terminal position-2 alanine. Vildagliptin binds with DPP-4 to produce a DPP-4 compound and inhibit its activity. It increases GLP21 concentration and promotes islet beta cells to produce insulin, while also lowering glucagon concentration, thus lowering blood sugar. Vildagliptin has not noticeable effects on weight.
- Adverse reactions Most common adverse reactions include headache, nasopharyngitis, coughing, constipation, dizziness and excess sweating. There have been very rare cases of hypotension, but it is unclear if conditions are related to this drug. Almost all research shows that the hypoglycemia occurrence rate while using Vildagliptin is similar to the rate when using a placebo. Control trials showed that compared to thiazolidinediones, Vildagliptin causes a similar occurrence rate of headaches, rashes, and other adverse reactions, but Vildagliptin has a lower occurrence rate of adverse reactions forcing patients to cease treatment and of severe adverse reactions.
- Description Vildagliptin, a DPP-4 inhibitor, was launched for the oral treatment of type 2 diabetes. Vildagliptin is the second DPP-4 inhibitor to reach the market behind sitagliptin, which was introduced in 2006. DPP-4 inhibitors act by slowing the inactivation of incretins, which are endogenous peptides involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase the synthesis and release of insulin from pancreatic βcells via intracellular signaling pathways involving cAMP. GLP-1 also lowers glucagon secretion from pancreatic α cells, which leads to reduced hepatic glucose production. However, although GLP-1 and GIP effectively lower blood glucose, they are short-lived as a result of rapid inactivation by the ubiquitous serine protease DPP-4. By inhibiting DPP-4, vildagliptin increases the concentration and duration of active incretin levels, which in turn results in increased insulin release and decreased glucagon levels in a glucose-dependent manner. Both vildagliptin and sitagliptin are potent, competitive, reversible inhibitors of DPP- 4 (IC50=3.5 and 18 nM, respectively), and they both show slow, tight-binding inhibition kinetics.
- Chemical Properties White Solid
- Originator Novartis (US)
- Uses The major metabolite of Vildagliptin
- Uses A metabolite of Vildagliptin
- Uses Vildagliptin (LAF-237) inhibits DPP?4 with IC50 of 2.3 nM
- Uses Labelled Vildagliptin. It is a new oral anti-hyperglycemic agent of a new dipeptidyl peptidase-IV (DPP-IV) inhibitor class of drugs. Antidiabetic.
- Definition ChEBI: Vildagliptin is an amino acid amide.
- brand name Galvus
-
Clinical Use
Dipeptidyl peptidase 4 inhibitor:
Treatment of type 2 diabetes mellitus in combination with other antidiabetic drugs - Side effects The most common adverse events reported in patients receiving vildagliptin included headache, nasopharyngitis, cough, constipation, dizziness, and increased sweating. Vildagliptin is not recommended for patients with liver impairment.
- Synthesis Vildagliptin is chemically derived in three steps starting from L-prolinamide via acylation with chloroacetyl chloride to produce 1-(chloroacetyl)-L-prolinamide, subsequent dehydration of the carboxamide group to the nitrile with trifluoroacetic anhydride, and condensation of the 1-(chloroacetyl)-L-prolinenitrile intermediate with 3-hydroxyadamantan-1-amine.
- target DDP-4
-
Metabolism
About 69
% of a dose of vildagliptin is metabolised, mainly by hydrolysis in the kidney to inactive metabolites. About 85
% of a dose is excreted in the urine (23
% as unchanged drug), and 15
% in the faeces. - References 1) Balas?et al.?(2007),?The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients;? J. Clin. Endocrinol. Metab.,?92?1249 2) Ahren?et al. (2004),?Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels and reduces glucagon levels in type 2 diabetes;? J. Clin. Endocrinol. Metab., 89?2078 3) Kosaraju?et al. (2013),?Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease;? J. Pharm. Pharmacol.,?65?1773 4) Shimizu?et al. (2012),?DPP4 inhibitor vildagliptin preserves? β-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice;? J. Mol. Endocrinol.,?49?125
Vildagliptin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(623)Suppliers
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
HangZhou RunYan Pharma Technology Co.,LTD.
- Tel: +8618882027439
- Email:sales2@runyanpharma.com
- Country:China
- ProdList:302
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-81138252<br/>+86-18789408387
- Email:1057@dideu.com
- Country:China
- ProdList:3922
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-81148696<br/>+86-15536356810
- Email:1022@dideu.com
- Country:China
- ProdList:3883
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
Henan Fengda Chemical Co., Ltd
- Tel:+86-371-86557731<br/>+86-13613820652
- Email:info@fdachem.com
- Country:China
- ProdList:20234
- Advantage:58
-
Supplier:
Shaanxi TNJONE Pharmaceutical Co., Ltd
- Tel:+86-17396673057
- Email:linda@tnjone.com
- Country:China
- ProdList:1143
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
Related articles
274901-16-5, VildagliptinRelated Search:
- Sitagliptin phosphate monohydrate ALTRENOGEST Vildagliptin Polyvinylpyrrolidone Acetylacetone Acetyl chloride Glycine Sitagliptin Betaine Adenine phosphate salt(1:1) N-ACETYL-L-TYROSINE ETHYL ESTER Acetylferrocene Sitagliptin phosphate Vildagliptin Carboxylic Acid vildagliptin Impurity E (S)-1-N-CBZ-2-CYANO-PYRROLIDINE D-Prolinamide Ascorbic Acid
- 274901-16-5
- Inhibitor
- API
- Metabolites & Impurities
- Isotope Labelled Compounds
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Heterocycles
- Chiral Reagents
- Amines
- Other APIs
- Galvus
- 高纯化学试剂
- 抑制剂
- 医药化学试剂
- 药靶配体
- FDA批准的配体
- 原料药-循环系统用药
- 自营化工原料
- 柏锦生物
- API原料药
- 医药体
- 化学试剂
- 化学原料
- 原料药及中间体
- 抗糖
- 医用原料
- 化工中间体
- 杂质对照品
- 医药、农药及染料中间体
- 有机中间体
- 化工原料药
- 药物杂质及中间体
- 标准品
- 中药对照品
- 医药原料
- 杂质对照品
- 维格列汀杂质
- 医药 治疗2-型糖尿病药物
- 原料
- 对照品-杂质对照品
- 医药原料药-原料药
- 中间体
- 蛋白酶
- 原料药
- 维格列汀(原料药)
- 医药原料药
- 研发中原料药
- 膦配体
- 小分子抑制剂
- 糖尿病
- 医药中间体
- 科研试剂
- C18H20F6N3O5P
- C23H33N3O8
- C17H22D3N3O2
- 维格列汀,10 MM DMSO 溶液
- 甲醇中维达列汀