ChemicalBook > CAS DataBase List > Sitagliptin
Sitagliptin
Description Mechanism of action Pharmacokinetics Indications Clinical Use Side effects Drug interactions
Sitagliptin
- CAS No.486460-32-6
- Chemical Name:Sitagliptin
- CBNumber:CB01449778
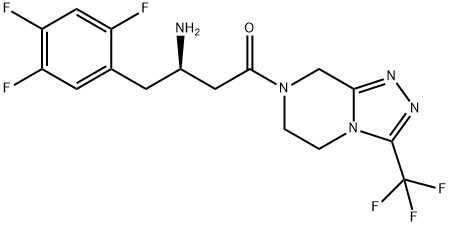
- Molecular Formula:C16H15F6N5O
- Formula Weight:407.31
- MOL File:486460-32-6.mol
Sitagliptin Property
- Melting point 114.1-115.7 °C
- Boiling point 529.9±60.0 °C(Predicted)
- Density 1.61±0.1 g/cm3(Predicted)
- storage temp. Store at -20°C
- solubility DMSO : ≥ 50 mg/mL (122.76 mM)
- pka 7.20±0.10(Predicted)
- form Solid
- color White to off-white
- InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1
- InChIKey MFFMDFFZMYYVKS-SECBINFHSA-N
- SMILES C(N1CCN2C(C(F)(F)F)=NN=C2C1)(=O)C[C@H](N)CC1=CC(F)=C(F)C=C1F
- CAS DataBase Reference 486460-32-6(CAS DataBase Reference)
- FDA UNII QFP0P1DV7Z
- ATC code A10BH01
- EPA Substance Registry System 1-Butanone, 3-amino-1-[5,6-dihydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazin-7(8H)-yl]-4-(2,4,5-trifluorophenyl)-, (3R)- (486460-32-6)
Safety
- Hazardous Substances Data :486460-32-6(Hazardous Substances Data)
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P305+P351+P338
Sitagliptin Chemical Properties,Usage,Production
-
Description
Sitagliptin is an orally-bioavailable selective DPP4 inhibitor that was discovered through the optimization of a class of β-aminoacid-derived DPP4 inhibitors. It lowers DPP4 activity in a sustained manner following once daily administration, preserves the circulating levels of intact GIP and GLP1 following meals in both acute and chronic studies and reduces blood glucose levels without significant increases in hypoglycaemia.
Sitagliptin phosphate (STG) is used to treat DM type 2 because it improves glycemic control by increasing the levels of active incretin hormones, GLP-1 (peptide-1) and GIP (glucose-dependent insulinotropic peptide). The activation of these incretins in β-pancreatic cells causes increased levels of cyclic adenosine monophosphate (cAMP) and intracellular calcium, with subsequent glucose-dependent insulin secretion (2). This hypoglycemic drug belongs to a new class called dipeptidyl peptidase IV inhibitors. STG was approved by the FDA in 2006. - Mechanism of action Sitagliptin prolongs the activity of proteins that increase the release of insulin after blood sugar rises, such as after a meal. Sitagliptin is a selective inhibitor of the enzyme dipeptidyl peptidase-4 (DPP-4), which metabolizes the naturally occurring incretin hormones glucagon-like peptide-1(GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) resulting in enhanced glucose-dependent insulin secretion from the pancreas and decreased hepatic glucose production. Since GLP-1 enhances insulin secretion in the presence of raised blood glucose levels, inhibiting DPP-IV activity will increase and prolong the action of GLP-1 by reducing its rate of inactivation in plasma. Sitagliptin reduces hemoglobin A1c (HbA1c), fasting and postprandial glucose by glucose-dependent stimulation of insulin secretion and inhibition of glucagon secretion. GLP-1 has other widespread effects including delaying gastric emptying, significantly reducing glucagons levels and possible central effects on the appetite.
- Pharmacokinetics Bioavailability of sitagliptin is approximately 87%. Halflife is between 8-14 hours. It is 38% bound to plasma proteins. It undergoes limited metabolism via CYP3A4 and CYP2C8. Elimination is mainly through urine.
- Indications Sitagliptin is approved by the FDA as an adjunct to diet and exercise to improve glycaemic control in patients with T2DM, either as a monotherapy, or in combination with metformin or a peroxisome proliferatoractivated receptor-γ agonist (for example, thiazolidinediones) when the single agent does not provide adequate glycaemic control.
- Clinical Use In October 2006, the U.S. Food and Drug Administration (FDA) approved sitagliptin as monotherapy and as add-on therapy to either of two other types of oral diabetes medications, metformin or thiazolidinediones to improve blood glucose control in patients with type 2 diabetes when diet and exercise are not enough. In March, 2007 it was approved in European Union. Sitagliptin is currently approved in 42 countries. The recommended dose of sitagliptin is 100 mg once daily. It may be taken with or without food. In April, 2007 FDA approved the combination product of sitaglibtin and metformin for type 2 diabetes. In clinical trials of 1-year duration, sitagliptin improved glycaemic control by reducing both fasting and postprandial glucose concentrations, leading to clinically meaningful reductions in glycosylated haemoglobin levels. Monotherapy with sitagliptin 100mg daily decreases mean HbA1c by 0.6- 0.79% (mean difference from placebo). When used in combination with metformin or pioglitazone, the mean reduction is HbA1c is 0.7% and 0.9% respectively. Sitagliptin is considered to be weight neutral and lipid neutral.
- Side effects In clinical trials, sitagliptin demonstrated an overall incidence of side effects comparable to placebo. The most common side effects in studies were upper respiratory tract infection, stuffy or running nose, sore throat, headache and diarrhea. The incidence of hypoglycemia with sitagliptin monotherapy was not significantly different than placebo. Pooled data from 2 monotherapy and 2 combination trials show that the incidence of hypoglycemia was 1.2% and 0.9% for sitagliptin 100mg and placebo respectively.
- Drug interactions Sitagliptin plasma concentrations may be increased modestly (approximately 68%), with cyclosporine which is not expected to be clinically important. Digoxin plasma levels may be increased slightly (approximately 18%), no dosage adjustment is recommended. Although sitagliptin is not as likely to cause hypoglycemia as some other oral diabetes medications, be careful while prescribing any other drug that can potentially lower blood sugar, such as: probenecid, nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin or other salicylates, sulfa drugs, a monoamine oxidase inhibitor (MAOI) or beta-blockers.
- Description Sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, is a first-in-class oral drug launched for the treatment of type 2 diabetes. It acts by slowing the inactivation of incretins, which are endogenous peptides involved in the physiologic regulation of glucose homeostasis. Incretin hormones, including glucagonlike peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. When blood glucose concentrations are normal or elevated,GLP-1 and GIP increase the synthesis and release of insulin from pancreatic b cells via intracellular signaling pathways involving cAMP. GLP-1 also lowers glucagon secretion from pancreatic αcells, which leads to reduced hepatic glucose production.
- Originator Merck (US)
- Uses Sitagliptin is a useful pharmaceutical drug. Could be useful for treating intestinal inflammation, diabetes, pre-?diabetes, impaired glucose tolerance, hepatitis, and?/or inflammatory liver disease.
- Uses Labeled Sitagliptin , intended for use as an internal standard for the quantification of Sitagliptin by GC- or LC-mass spectrometry.
- Definition ChEBI: Sitagliptin is a triazolopyrazine that exhibits hypoglycemic activity. It has a role as a serine proteinase inhibitor, a hypoglycemic agent, an EC 3.4.14.5 (dipeptidyl-peptidase IV) inhibitor, an environmental contaminant and a xenobiotic. It is a triazolopyrazine and a trifluorobenzene.
- brand name Januvia
- Clinical Use Treatment of type 2 diabetes in combination with metformin or a thiazolidinedione
-
Metabolism
Sitagliptin undergoes minimal metabolism, mainly by the
cytochrome P450 isoenzyme CYP3A4, and to a lesser
extent by CYP2C8.
Renal excretion of sitagliptin involves active tubular secretion; it is a substrate for organic anion transporter-3 and P-glycoprotein.
Sitagliptin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(538)Suppliers
-
Supplier:
Changzhou Ruiming Pharmaceutical Co., LTD
- Tel: +8613901491351
- Email:13901491351@126.com
- Country:United States
- ProdList:1
- Advantage:58
-
Supplier:
Anhui Ruihan Technology Co., Ltd
- Tel: +8617756083858
- Email:daisy@anhuiruihan.com
- Country:China
- ProdList:973
- Advantage:58
-
Supplier:
HangZhou RunYan Pharma Technology Co.,LTD.
- Tel: +8618882027439
- Email:sales2@runyanpharma.com
- Country:China
- ProdList:302
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Shanghai UCHEM Inc.
- Tel:+862156762820<br/>+86-13564624040
- Email:sales@myuchem.com
- Country:China
- ProdList:7906
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
Sinoway Industrial co., ltd.
- Tel:0592-5800732;<br/>+8613806035118
- Email:xie@china-sinoway.com
- Country:China
- ProdList:987
- Advantage:58
-
Supplier:
Hebei Dangtong Import and export Co LTD
- Tel:+86-86-4001020630<br/>+8619831957301
- Email:admin@hbdangtong.com
- Country:China
- ProdList:998
- Advantage:58
Related articles
486460-32-6, SitagliptinRelated Search:
- Terazosin hydrochloride Sitagliptin phosphate monohydrate 5-Aza-2'-deoxycytidine Prazosin 3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine Sitafloxacin 6-Aminocaproic acid Cilostazol Doxazosin 3-Aminophenol Tris(hydroxymethyl)aminomethane Alfuzosin Doxazosin mesylate ALTRENOGEST Sitagliptin phosphate Alfuzosin hydrochloride Glycine Cilastatin
- 486460-32-6
- API
- Pharmaceutical intermediate
- Heterocycles
- 二肽基肽酶-4(DPP-4)抑制剂
- 化学原料药
- 无机化工
- 抑制剂
- FDA批准的配体
- 医药原料药API
- 标准品
- 化学试剂
- 医药体
- 药物
- 医药-医药
- 日用化学品
- 原料药及中间体
- 化药杂质-西他列汀
- 化工中间体
- 原料药
- 医药化工类
- 原料
- 化工原料药
- 医药原料
- 医用原料
- 化工原料
- 医药原料药-科研原料
- 对照品-杂质对照品
- 杂质对照品
- 西他列汀系列
- 细胞生物学试剂
- 激素类科研原料药
- 医药中间体
- 小分子抑制剂
- 西他列汀中间体
- 中间体
- 西他列汀
- C16H15F6N5O
- 1011232-08-8
- 西他列汀及其中间体
- 西他列汀 SITAGLIPTIN
- 西他列汀,西他列汀及其中间体
- SIT-B
- 乙腈中西他列汀
- 西他列汀盐酸盐-D4
- 甲醇中西他列汀
- 化合物 JANUMET
- 西他列汀相关杂质
- 西他列汀|||MK0431|||西格列汀
- (R)-3-氨基-1-(3-(三氟甲基)-5,6-二氢-[1,2,4]三氮唑并[4,3-A]吡嗪-7(8H)-基)-4-(2,4,5-三氟苯基)丁-1-酮
- 西他列汀API及中间体
- 西格列汀游离碱
- 西格列汀(碱)
- (3R)-3-氨基-1-[3-(三氟甲基)-5,6,7,8-四氢-1,2,4-三唑并[4,3-A]吡嗪-7-基]-4-(2,4,CHEMICALBOOK5-三氟苯基)丁-1-酮
- 西他列汀BASE(碱)
- 西他列汀 (无水)
- 西格列汀BASE
- 谷胱甘肽, 西他列汀