ChemicalBook > CAS DataBase List > Daunorubicin hydrochloride
Daunorubicin hydrochloride
Daunorubicin hydrochloride
- CAS No.23541-50-6
- Chemical Name:Daunorubicin hydrochloride
- CBNumber:CB7113022
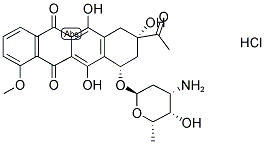
- Molecular Formula:C27H29NO10.ClH
- Formula Weight:563.98
- MOL File:23541-50-6.mol
Daunorubicin hydrochloride Property
- Melting point 190°C (dec)
- alpha D20 +248 ±5° (c = 0.05-0.1 in methanol)
- storage temp. Inert atmosphere,2-8°C
- solubility Freely soluble in water and in methanol, slightly soluble in alcohol, practically insoluble in acetone.
- form solid
- color red to deep red
- Water Solubility Soluble in water (50 mM)
- Sensitive Hygroscopic
- λmax 477nm(H2O)(lit.)
- Merck 14,2832
- BRN 4229221
- Stability Stability
- InChIKey GUGHGUXZJWAIAS-SMLRDAARNA-N
- SMILES C1=CC2C(=O)C3=C(O)C4C[C@](C(C)=O)(O)C[C@H](O[C@@H]5O[C@@H](C)[C@@H](O)[C@@H](N)C5)C=4C(O)=C3C(=O)C=2C(OC)=C1.Cl |&1:10,16,18,20,22,24,r|
- NCI Dictionary of Cancer Terms Cerubidine; daunomycin hydrochloride; daunorubicin hydrochloride
- FDA UNII UD984I04LZ
- Proposition 65 List Daunorubicin Hydrochloride
- NCI Drug Dictionary Cerubidine
- EPA Substance Registry System Daunorubicin hydrochloride (23541-50-6)
- UNSPSC Code 41116107
- NACRES NA.77
Safety
- Hazard Codes :Xn,Xi
- Risk Statements :22-40-42/43-36/37/38-20/21/22
- Safety Statements :22-36/37-45-37/39-36-26
- RIDADR :UN 2811 6.1/PG 3
- WGK Germany :3
- RTECS :HB7878000
- F :3-10
- HazardClass :6.1(b)
- PackingGroup :III
- HS Code :29419059
- Toxicity :LD50 in mice (mg/kg): 26 i.v. (DiMarco, 1969)
-
NFPA 704:
0 2 0
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H301-H341-H351-H360FD
- Precautionary statements P201-P202-P264-P270-P280-P301+P310
Daunorubicin hydrochloride Price
More Price(6)
- Brand: Sigma-Aldrich(India)
- Product number: D8809
- Product name : Daunorubicin hydrochloride
- Purity: meets USP testing specifications
- Packaging: 1MG
- Price: ₹3972.78
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: D8809
- Product name : Daunorubicin hydrochloride
- Purity: meets USP testing specifications
- Packaging: 10MG
- Price: ₹17449.9
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 30450
- Product name : Daunorubicin hydrochloride
- Purity: ≥90% (HPLC)
- Packaging: 5MG
- Price: ₹12621.95
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 30450
- Product name : Daunorubicin hydrochloride
- Purity: ≥90% (HPLC)
- Packaging: 25MG
- Price: ₹28718.73
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: D4532
- Product name : Daunorubicin Hydrochloride
- Purity:
- Packaging: 20MG
- Price: ₹4700
- Updated: 2022/05/26
- Buy: Buy
Daunorubicin hydrochloride Chemical Properties,Usage,Production
- Description Daunorubicin HCl (23541-50-6) is an antitumor antibiotic used in the treatment of acute myeloid leukemias.1?Induces DNA damage by intercalation.2?Induces apoptosis in a variety of cell lines.3?Inhibition of autophagy with chloroquine enhances daunorubicin-induced apoptosis in K562 cells.4?Cell permeable.
- Chemical Properties Dark Red Crystals
- Originator Cerubidine,Rhone-Poulenc Rorer,France
- Uses A DNA intercalator which may suppress acute leukemia proliferation
- Uses anti-neoplastic LD50 in mice 26 mg/kg
- Uses Antineoplastic;DNA intercaling
- Uses Anthracycline antibiotic related to the rhodomycins. Antineoplastic
- Definition ChEBI: Daunorubicin hydrochloride is an anthracycline.
-
Manufacturing Process
A 170 liter fermentation vessel is charged with:
Corn steep
2.400 kg
Sucrose
3.600 kg
Calcium carbonate
0.900 kg
Ammonium sulphate
0.240 kg
Water
to 100 liters
This culture medium has a pH of 6.15. It is sterilised by passage of steam at 122°C for 40 minutes. After cooling, the volume of the broth is 120 liters and the pH is 7.20. The medium is then seeded with 200 cc of a culture of the strain Streptomyces 31723. The culture is carried out for 27 hours at 26°- 27°C with agitation and aeration with sterile air. It is then suitable for seeding the production culture. The production culture is carried out in an 800 liter formentation vessel charged with the following:
Soya flour 20 kg
Distillers' solubles 2.500 kg
Soya oil 2.500 liters
Soya oil 2.500 liters
Sodium chloride 5 kg
Water to 465 liters
Starch 10 kg
The pH of the medium thus obtained is adjusted to 7.20 with concentrated sodium hydroxide solution (400 cc). The medium is then sterilised by the passage of steam at 122°C for 40 minutes. After cooling, the volume of the broth is 500 liters and the pH is 6.75. It is then seeded with 50 liters of the culture from the 170 liter fermentation vessel. Culture is carried out at 28°C for 67 hours with agitation and aeration with sterile air. The pH of the medium is then 7.40 and the volume of the fermentation culture is 520 liters. The quantitiy of antibiotic present in the medium is 29 μ/cc.
The above fermentation culture (520 liters; activity 29 μ/cc) is placed in a vessel equipped with an agitator and the pH is adjusted to 1.8 with a concentrated solution of oxalic acid. Agitation is carried out for one hour and a filtration adjuvant (20 kg) then added. The mixture is filtered on a filter-press and the filter-cake washed with water (100 liters) acidified to pH 2 with oxalic acid. The filtrate (612 liters) is treated with concentrated sodium hydroxide solution until the pH is 4.5. The filtrate is then passed through a column containing Amberlite IRC 50 in hydrogen form (20 liters; diameter of column 15.2 cm, height of column 200 cm, height of resin at rest in column 110 cm). The filtrate passes through the bed of Amberlite from base to top at a rate of 40 liters/hour. The column is then washed with water (100 liters) at a rate of 50 liters/hour circulating from base to top and then with methanol (containing 10% water; 75 liters) circulating from top to base at a rate of 50 liters/hour. The washings are discarded and the column is then eluted with a solution having the following composition (per liter):
The eluate (100 liters), which contains the major part of the antibiotic, is concentrated under reduced pressure at 35°C to 10 liters. The concentrate is extracted at pH 7.5 with chloroform (2 times 5 liters). The chloroformic extract is adjusted to pH 4 with a solution of acetic acid in chloroform (10:100 by volume) and then concentrated at 30°C under reduced pressure to 100 cc. The antibiotic is precipitated by the addition of hexane (1 liter), separated, washed and dried to give an amorphous red powder (9 g) of activity 1,400 μ/mg.
The crude antibiotic (17.1 g) obtained as above described (activity 1530 μ/mg) is dissolved with stirring in a mixture of methylene chloride (1.5 liters), carbon tetrachloride (0.3 liters) and water (1.8 liters). The pH is then adjusted
9865 RP (500 mg), obtained as above described, is dissolved in normal sulphuric acid (100 cc) and the solution obtained is heated for 20 minutes on a water-bath. After cooling and extracting with ethyl acetate (3 times 200 cc), the organic extract is dried over anhydrous sodium sulphate, filtered and concentrated to a small volume, giving, after filtering, washing and drying, crystals (218.5 mg). These crystals (150 mg) are dissolved in chloroform (3 cc) and benzene (1.5 cc) and the solution obtained is chromatographed on 20 sheets of Arches No. 310 paper impregnated with a solution of acetone containing 20% formamide, and developed for 90 minutes by means of a 2:1 mixture of chloroform and benzene saturatd with formamide. The principal zone of Rf = 0.86 is cut out of each of the 20 sheets and the 20 zones thus cut out are comminuted in a mixer in the presence of methanol. The mixture obtained is filtered, concentrated, and water (10 volumes) added. The precipitate obtained is filtered off, washed and dried under reduced pressure to give crystals (120 mg). These crystals (170 mg) are dissolved in dioxan containing 20% water (15 cc) and water acidified to pH 4 with 0.1 N hydrochloric acid is added dropwise. The crystals formed are filtered off, washed and dried, thus giving the aglycone of 9865 RP (130 mg) in the form of orange-red needles, having a first melting point at 160°C and a second at 225°C-230°C.
Daudorubicin can be prepared by gene ingineering methods also. to 3 by the addition of normal hydrochloric acid (8 cc). After decanting, the aqueous phase is treated with methylene chloride (7 liters) and 0.1 N sodium hydroxide solution (200 cc) to give a pH of 7.5. After decanting, the aqueous phase is again extracted at pH 7.5 with methylene chloride (3.5 liters). The methylene chloride extracts are combined and concentrated to 100 cc. After the addition of hexane (1 liter) to the concentrate, a product precipitates which is filtered off, washed and dried at 30°C under reduced pressure to give the antibiotic 9865 RP (9.15 g) in the form of an amorphous orange-red powder of activity 2180 μ/mg. - brand name Cerubidine (Bedford); Cerubidine (Sanofi Aventis); Cerubidine (Wyeth).
- Therapeutic Function Antineoplastic
-
General Description
Daunorubicin is available in 20- and 100-mg vials for reconstitution.The agent is given intravenously for the treatmentof acute nonlymphocytic and lymphocytic leukemia. Otherunlabeled uses include CML and Kaposi sarcoma. In general,its use is more limited than that of doxorubicin.Daunorubicin lacks the hydroxyl groups found at C-14 ofdoxorubicin. This leads to an increase in the amount of thealcohol metabolite daunorubicinol (active) arising as a resultof reduction of the side chain ketone. This, however, doesnot appear to lead to a significant increase in the occurrenceof cardiotoxicity compared with doxorubicin. The mechanismsof resistance and toxicities of daunorubicin are similarto that of doxorubicin; the major difference betweenthe two agents being the spectrum of cancers that they areused treat.
A liposomal form of daunorubicin is also availableknown as DaunoXome. The drug offers the same advantagesas those seen for the liposomal form of doxorubicin. - General Description Orange-red powder. Thin red needles decomposing at 188-190°C. An anti-cancer drug.
- Air & Water Reactions Water soluble.
- Reactivity Profile Daunorubicin hydrochloride may emit toxic oxides of nitrogen when heated.
- Biological Activity Anticancer agent that is clinically used to treat nonlymphocytic leukaemia. Inhibits RNA and DNA synthesis and causes DNA fragmentation in vivo .
- Biochem/physiol Actions Potent anticancer agent. Inhibits DNA and RNA synthesis as sequence specific ds-DNA intercalating agent.
- Pharmacokinetics Like Doxil (the liposomal formulation of doxorubicin), DaunoXome is indicated for use in AIDS-related Kaposi's sarcoma and is administered IV at a dose of 40 mg/m2 every 2 weeks. The pharmacokinetic profiles of Doxil and DaunoXome are similar.
- Clinical Use Daunorubicin is administered IV at a dose of 45 mg/m2 for the treatment of lymphocytic and nonlymphocytic leukemia.
- Metabolism The 18.5-hour terminal half-life of daunorubicin is approximately half that of doxorubicin, and the terminal half-life of the active daunorubicinol metabolite is 26.7 hours. Excretion is approximately 40% biliary and 25% urinary.
- storage +4°C
- References 1) Laurent and Jaffrezou (2001),?Signaling pathways activated by daunorubicin; Blood?98?913 2) Yang et al. (2014), Doxorubicin, DNA torsion, and chromatin dynamics; Biochim.Biophys.Acta, 1845 84 3) Masquelier?et al.?(2004),?Relationship between daunorubicin concentration and apoptosis induction in leukemic cells; Biochem.Pharmacol.?67?1047 4) Han?et al.?(2011),?Autophagy inhibition enhances daunorubicin-induced apoptosis in K562 cells; PLoS One?6?e28491
Daunorubicin hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
Global(543)Suppliers
-
Supplier:
Protheragen-ING
- Tel: +16313385890
- Email:info@protheragen-ing.com
- Country:United States
- ProdList:3868
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
Apeloa production Co.,Limited
- Tel: +8619933239880
- Email:admin@apcl.com.cn
- Country:China
- ProdList:861
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co Ltd
- Tel:+86-16264648883<br/>+86-16264648883
- Email:niki@zlchemi.com
- Country:China
- ProdList:7245
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shanghai Yingrui Biopharma Co., Ltd.
- Tel:+86-21-33585366 - 03@
- Email:sales03@shyrchem.com
- Country:CHINA
- ProdList:738
- Advantage:60
-
Supplier:
Hubei XinRunde Chemical Co., Ltd.
- Tel:+8615102730682
- Email:bruce@xrdchem.cn
- Country:CHINA
- ProdList:566
- Advantage:55
23541-50-6, Daunorubicin hydrochlorideRelated Search:
- Acetyl coenzyme A sodium salt 6-Aminocaproic acid ALTRENOGEST Doxorubicin hydrochloride Doxycycline hyclate Clindamycin hydrochloride Tetrahydro-4H-pyran-4-one ACLARUBICIN HYDROCHLORIDE Acetylacetone SPECTINOMYCIN DIHYDROCHLORIDE Centchroman Tris(hydroxymethyl)aminomethane Epirubicin hydrochloride Spectinomycin dihydrochloride pentahydrate Topotecan hydrochloride Glycine Lincomycin hydrochloride Hydroxydiethylphenamine
- Coronavirus
- Anti-cancer&immunity
- API
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Heterocycles
- Chiral Reagents
- Carbohydrates & Derivatives
- Antitumour
- 原料中间体
- 中药标准品,对照品
- 其他中间体
- 化学原料药
- 杂质对照品
- 抑制剂
- 生化试剂
- 生物医药-医药原料
- 化工试剂
- 兽用原料
- 试剂
- 日用化工
- 化学原料
- 医药原料及中间体
- 科研原药系列
- 细胞生物学试剂
- 中药对照品
- 对照品-中药对照品
- 原料药
- 中间体类
- 原料
- 化工原料
- 医药、农药及染料中间体
- 化学试剂-科研试剂
- 日用化学品
- 化学试剂
- 精细化工原料
- 抗癌药
- 科研试剂
- BICINS比星类产品(Anti-cancer)
- 生物活性小分子
- 细胞生物学
- 医药中间体
- 小分子抑制剂
- 发酵类
- 抗肿瘤类
- 医药原料,中间体
- 医药原料
- 医药原料药
- 抗生素
- 原料药【仅供科研】
- Antibiotics A-F
- Antibiotics A to Z
- Antibiotics
- BioChemical
- Cell Biology
- Cell Signaling and Neuroscience
- DNA-RNA Transcription Regulators
- Gene Regulation and Expression