ChemicalBook > CAS DataBase List > iodoxamic acid
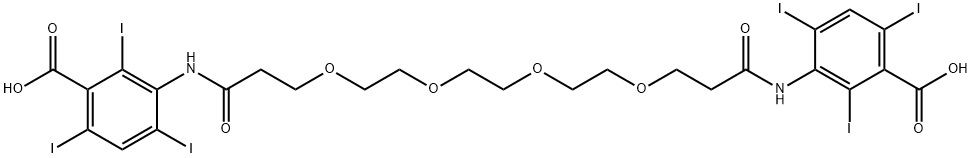
iodoxamic acid
iodoxamic acid
- CAS No.31127-82-9
- Chemical Name:iodoxamic acid
- CBNumber:CB6937203
- Molecular Formula:C26H26I6N2O10
- Formula Weight:1287.92
- MOL File:31127-82-9.mol
iodoxamic acid Property
- Boiling point 977.7±65.0 °C(Predicted)
- Density 2.423±0.06 g/cm3(Predicted)
- pka pKa 1.8/2.8(H2O,t = 25) (Uncertain)
- FDA UNII NS1Y283HW4
- ATC code V08AC01
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
iodoxamic acid Chemical Properties,Usage,Production
- Originator Endobil,Bracco
- Uses Diagnostic aid (radiopaque medium).
- Definition ChEBI: Iodoxamic acid is an organic molecular entity.
-
Manufacturing Process
148.5 g 4,7,10,13-tetraoxahexadecane-1,16-dinitrile (U.S. Patent No.
2,401,607) was added to a solution of 232 g (2.45 mol) concentrate sulfuric
acid in 290 ml absolute ethanol at 15°C. The mixture was heated at reflux for
15 hours, cooled and poured into 1000 g ice and 250 g ammonium sulfate. It
was extracted with methylene chloride, dried and a solvent was removed in
vacuum. The residue was distilled to give 4,7,10,13-tetraoxahexadecane-1,16-
dicarbonic acid dimethyl ester; BP: 190°-195°C/0.005 mm Hg.
1 mol above prepared diester was saponificated with equivalent of NaOH in water. The reaction mixture was heated for 90 minutes. On cooling it was extracted with ether and the water layer was evaporated to dryness. The residue was washed with acetone. The obtained disodium salt of 4,7,10,13- tetraoxahexadecane-1,16-dicarbonic acid (yield 100%; MP: 102°-104°C) was acidified with calculated quantity of HCl to give the dicarbonic acid. The solvent was evaporated to dryness. Acetone was added to the residue for removing a by-product (sodium chloride) by filtration. Acetone was evaporated and the residue was extracted with ether, dried and evaporated. The residual liquid was 4,7,10,13-tetraoxahexadecane-1,16-dicarbonic acid.
100 ml thionyl chloride was cautious added to 56 g above prepared diacid and heated at 40°-50°C and excess thionyl chloride was distilled in vacuum. The residue was the 4,7,10,13-tetraoxahexadecane-1,16-dicarbonic acid dichloride. The desired iodoxamic acid was prepared from above dichloride and 3-amino-2,4,6-trijode benzoic acid, in dimethylacetamide - brand name Endobil (Bracco Industria Chimica S.p.A.,Italy); Endomirabil (Bracco Industria Chimica S.p.A., Italy); Videocolangio (Bracco Industria Chimica S.p.A., Italy).
- Therapeutic Function Diagnostic aid
iodoxamic acid Preparation Products And Raw materials
Raw materials
Preparation Products
Global(8)Suppliers
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: +8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
- Supplier: Baoji Didu Pharmaceutical and Chemical Co., Ltd
- Tel:029-02961856358<br/>15829236573
- Email:1035@dideu.com
- Country:China
- ProdList:10010
- Advantage:58
31127-82-9, iodoxamic acidRelated Search:
- C26H26I6N2O10
- 碘沙酸
- 31127-82-9
- iodoxamic acid USP/EP/BP
- Benzoic acid, 3,3'-[(1,16-dioxo-4,7,10,13-tetraoxahexadecane-1,16-diyl)diimino]bis[2,4,6-triiodo-
- 3-[3-[2-[2-[2-[3-(3-carboxy-2,4,6-triiodoanilino)-3-oxopropoxy]ethoxy]ethoxy]ethoxy]propanoylamino]-2,4,6-triiodobenzoic acid
- iodoxamic acid
- Iodoxamic
- SQ-21982
- BC-17
- B-10610
- 3,3'-[Ethylenebis(oxyethyleneoxyethylenecarbonylimino)]bis(2,4,6-triiodobenzoic acid)
- 3,3'-[(1,16-Dioxo-4,7,10,13-tetraoxahexadecane-1,16-diyl)diimino]bis(2,4,6-triiodobenzoic acid)