ChemicalBook > CAS DataBase List > ISOFENPHOS
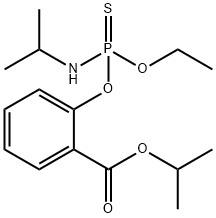
ISOFENPHOS
ISOFENPHOS
- CAS No.25311-71-1
- Chemical Name:ISOFENPHOS
- CBNumber:CB6667609
- Molecular Formula:C15H24NO4PS
- Formula Weight:345.39
- MOL File:25311-71-1.mol
ISOFENPHOS Property
- Melting point -12℃
- Boiling point bp0.01 120°
- Density 1.131 g/cm3 (20 ºC)
- vapor pressure 2.2 x 10-4 Pa (20 °C)
- storage temp. 0-6°C
- solubility Chloroform (Slightly), DMSO (Slightly)
- Water Solubility 18 mg l-1 (20 °C)
- pka -0.30±0.70(Predicted)
- form Oil
- color Colourless
- CAS DataBase Reference 25311-71-1(CAS DataBase Reference)
- EWG's Food Scores 3
- FDA UNII 0514MAW53A
- Pesticides Freedom of Information Act (FOIA) Isofenphos
- EPA Substance Registry System Isofenphos (25311-71-1)
Safety
- Hazard Codes :T,N,Xn,F
- Risk Statements :24/25-50/53-52/53-36-20/21/22-11
- Safety Statements :36/37-45-60-61-26-16
- RIDADR :3018
- WGK Germany :2
- RTECS :VO4395500
- HazardClass :6.1(a)
- PackingGroup :I
- HS Code :29299090
- Hazardous Substances Data :25311-71-1(Hazardous Substances Data)
- Toxicity :LD50 orally in rats, mice (mg/kg): 30-40, 90-130 (Homeyer)
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H225-H302+H312+H332-H319-H412
- Precautionary statements P210-P273-P280-P305+P351+P338
ISOFENPHOS Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 32860
- Product name : Isofenphos solution
- Purity: 100?μg/mL in acetonitrile, PESTANAL?, analytical standard
- Packaging: 2ML
- Price: ₹8019.9
- Updated: 2022/06/14
- Buy: Buy
ISOFENPHOS Chemical Properties,Usage,Production
- Chemical Properties Isofenphos is a colorless oil at room temperature. It is sparingly soluble in water, but soluble in cyclohexone, toluene, acetone, and diethyl ether. The US EPA has grouped isofenphos under RUP, which indicates that qualifi ed, certifi ed, and trained workers are required in the safety management of isofenphos. It is used on turf and ornamental trees and shrubs to control white grubs, mole crickets, and other insects, such as soil-dwelling insects, cabbage root fl ies, corn roundworms, and wire worms.
- Uses Isofenphos is used as a systemic insecticide to control cabbage root flies, carrot flies, onion flies and other soil insects in vegetables, rape, maize, bananas, sugar cane and other crops.
- Uses Isofenphos is an organophosphorus insecticide used in soil to control leaf-eating and soil-dwelling pests in vegetables, fruits, turf and field crops.
- Uses Insecticide.
- Definition ChEBI: Isofenphos is an organothiophosphate insecticide, an organic phosphonate, a phosphonic ester, a member of salicylates and an isopropyl ester. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor and an agrochemical. It is functionally related to an isopropyl salicylate.
- General Description Colorless oil. Non corrosive. Used as an insecticide.
- Air & Water Reactions Hydrolyzed by alkali solution.
- Reactivity Profile Organothiophosphates, such as ISOFENPHOS, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
- Health Hazard Exposures to isofenphos are highly toxic to animals and humans. Acute and prolonged exposures to high concentrations of isofenphos has caused poisoning with symptoms such as increased secretions, diffi culty in breathing, diarrhea, urination, pupil contraction, slowness of the heart, convulsions, and coma. Isofenphos in combination with malathion cause severe poisoning in humans.
-
Environmental Fate
Soil. Rapidly degraded by microbes via oxidative desulfuration in soils forming isofenphos
oxon (Abou-Assaf et al., 1986; Abou-Assaf and Coats, 1987; Somasundarum et al.,
1989), isopropyl salicylate and carbon dioxide (Somasundaram et al., 1989). The formation
of isofenphos oxon is largely dependent upon the pH, moisture and temperature of the
soil. The degradation rate of isofenphos decreased with a decrease in temperature (35°C
>25°C >15°C), moisture content (22.5% >30% >15%) and in acidic and alkaline soils
(pH 6 and 8 >pH 7). After isofenphos was applied to soil at a rate of 1,12 kg/ai/ha, concentrations
of 8.3, 7.2, 5.1 and 1.0 ppm were found after 5, 21, 43 and 69 days, respectively.
Following a second application, 4.9, 1.55, 0.25 and 0.10 ppm of isofenphos were found
after 5, 21, 43 and 69 days, respectively (Abou-Assaf and Coats, 1987).
A pure culture of Arthrobacter sp. was capable of degrading isofenphos at different soil concentrations (10, 50 and 100 ppm) in less than 6 hours. In previously treated soils,isofenphos could be mineralized to carbon dioxide by indigenous microorganisms (
Plant. Two weeks following application to thatch, 65% of the applied amount was present (Sears et al., 1987).
Surface Water. In estuarine water, the half-life of isofenphos ranged from 9.8 to 11.9 days (Lacorte et al., 1995).
Photolytic. Irradiation of an isofenphos (500 mg) in hexane and methanol (100 mL) using a high pressure mercury lamp (l = 254–360 nm) for 24 hours yielded the following products: isofenphos oxon and O-ethyl hydrogen-N-isopropylphosphoroamidothioa - Metabolic pathway 14C-Isofenphos is bioactivated by mixed-function oxidases that ultimately give N-desisopropylisofenphos oxon, a product with 2300-fold greater inhibitory potency than isofenphos oxon towards housefly head acetylcholinesterase.271 By housefly and the cuperous chafer, isofenphos is metabolized to give detoxified metabolites without the bioactivated N- desisopropylisofenphos oxon. No difference in kinds of metabolite is found between the two insects. When rats are administered 14C-isofenphos, most of the radioactivity is recovered from the water fraction of the urine and feces. Aminoisofenphos and isofenphos oxon are detected in the benzene fraction, and the other six metabolites are identified as water-soluble metabolites which include O-ethylhydrogen phosphoramidate and O-ethylhydrogen isopropylphosphoramidothioate. Water-soluble metabolites are predominantly formed through cleavage of the P-O-aryl linkage.
- Metabolism Main degradation route is cleavage of the P?O-aryl ester linkage through oxidative desulfuration to isofenphos oxon followed by hydrolysis and oxidative dearylation from isofenphos. In plant, the major metabolites are salicylic acid and dihydroxybenzoic acid.
- Toxicity evaluation The acute oral LD50 for rats is about 20 mg/kg. Inhalation LC50 (4 h) for rats is 0.3–0.5 mg/L air. NOEL (2 yr) for rats 1 mg/kg diet (0.05 mg/kg/d). ADI is 1 μg/kg. In mammals, administered isofenphos is rapidly metabolized and eliminated; almost 95% is excreted within 24 h in the urine and feces. The active metabolite is des-N-isopropylisofenphos oxon.
- Degradation Isofenphos is stable to hydrolysis with a DT50 of >1 year at pH values from 4 to 9. Photodegradation on soil surfaces in the laboratory was extremely rapid but under natural light conditions photolysis was slower (PM).
ISOFENPHOS Preparation Products And Raw materials
Raw materials
Preparation Products
Global(0)Suppliers
25311-71-1, ISOFENPHOSRelated Search:
- Ethyl 2-hydroxybenzoate Isocarbophos Isofenphos-methyl PESTANAL,ISOFENPHOS-METHYL ISOFENPHOS OXON STANDARD,ISOFENPHOS-OXON,ISOFENPHOS-O-ANALOGUE O,O-Dimethyl phosphoramidothioate ISOFENPHOS Isopropyl salicylate ISOFENPHOS-DES-N-ISOPROPYL methyl isofenphos ISOFENPHOS-DES-N-ISOPROPYL-OXON,ISOFENPHOS-DES-N-ISOPROPYL-O-ANALOGUE Isofenphos methyl E.C.,synergistic Isofenphos methyl granule Isofenphos methyl E.C. ISOFENPHOS-DES-N-ISOPROPYL 100MG [R] ISOFENPHOS-DES-N-ISOPROPYL-O-ANALOGUE 10NG/UL IN CYCLOHEXANE 5ML [R] ISOFENPHOS SOLUTION 100UG/ML IN ACETONITRILE 1ML ISOFENPHOS-DES-N-ISOPROPYL 100UG/ML IN HEXANE 1ML [R] ISOFENPHOS SOLUTION 100UG/ML IN TOLUENE 1ML
- Organophorous
- IPesticides
- Insecticides
- Analytical Standards
- Alphabetic
- Pesticides&Metabolites
- H-MAnalytical Standards
- Alpha sort
- 分析标准品
- 杀虫剂
- 有机氯杀虫剂
- 农残、兽药及化肥类
- C15H24NO4PS
- 异柳磷溶液标准物质
- 异柳磷溶液
- 甲醇中异柳磷溶液标准物质
- 标准样品/丙酮中异柳磷
- 标准物质/甲醇中异柳磷
- 异柳磷-D7
- 甲醇中异柳磷
- 丙酮中异柳磷
- 丙酮中异柳磷溶液,100ΜG/ML
- ISOFENPHOS 100NG/UL于环己烷 标准品
- ISOFENPHOS标准品
- 丙酮中异柳磷标准品
- 异柳磷 标准品
- 异丙胺磷 溶液
- 异柳磷溶液,100PPM
- 异柳磷检测标样(100 ΜG/ML 溶剂为甲醇)
- 异丙胺磷标准品
- 丙胺磷粉剂、颗粒剂
- 异芬硫磷
- O-乙基-O-(2-异丙氧羰基)-苯基-N-异丙基硫逐磷酰胺
- 异柳磷2号与异丙醇ISOFENPHOS&
- O-甲基-0-(邻异丙氧基羰基苯基)硫代磷酰酰胺
- 丙胺磷
- 异柳磷
- 甲基异柳磷(阿玛择)
- 2-(乙氧基-异丙基氨基-硫代磷酰氧)苯甲酸异丙酯
- 25311-71-1
- XA14420000CY Isofenphos 100μg/mLin Cyclohexane
- Isofenphos 100 μg/ml Toluene
- Isofenphos 100 μg/ml Acetonitrile
- Isofenphos 10.0 μg/ml Ethyl acetate
- C14420000 Isofenphos
- Isofenphos in Methanol
- Isofenphos in Acetone
- Isofenphos Solution in Acetone, 100μg/mL
- Benzoic acid, 2-[[ethoxy[(1-methylethyl)amino]phosphinothioyl]oxy]-, 1-methylethyl ester
- Isofenphos Reference Material
- Isofenphos Solution, 100ppm
- Pyfron 6
- Pryfon 6
- Bayer 92114
- le-mat
- isopropylsalicylateo-esterwitho-ethylisopropylphosphoramidothioate
- isopropyl-phosphoramidothioicacio-ethylo-(2-isopropoxycarbonylphenyl)
- Isopropyl 2-([ethoxy(isopropylamino)phosphorothioyl]oxy)benzoate