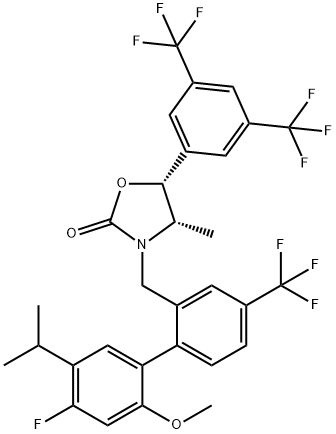
Anacetrapib
- CAS No.875446-37-0
- Chemical Name:Anacetrapib
- CBNumber:CB62487713
- Molecular Formula:C30H25F10NO3
- Formula Weight:637.51
- MOL File:875446-37-0.mol
- Boiling point 555.3±50.0 °C(Predicted)
- Density 1.345
- storage temp. Store at -20°C
- solubility ≥31.9 mg/mL in DMSO; insoluble in H2O; ≥86.6 mg/mL in EtOH
- form solid
- pka -2.30±0.60(Predicted)
- color White to off-white
- FDA UNII P7T269PR6S
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H335-H302-H319
- Precautionary statements P264-P270-P301+P312-P330-P501-P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362
Anacetrapib Chemical Properties,Usage,Production
- Uses An orally active and potent cholesterol ester transfer protein (CETP) inhibitor for the treatment of atherosclerosis, in particular dyslipidemia.
- Biological Activity anacetrapib, also known as mk-0859, is a potent and selective inhibitor of cholesteryl ester transfer protein (cetp) that exhibits strong inhibition against cetp-mediated transfer of cholesteryl esters and triglycerides with values of inhibition constant ic50 of 16 nm and 29 nm respectively. anacetrapib is mainly metabolized by cyp3a4 through o-demethylation, hydroxylation on the biphenyl moiety and hydroxylation on the isopropyl side chain resulting in three oxidative metabolites m1, m2 and m3 respectively. anacetrapib is being investigated for the treatment of primary hypercholesterolemia and mixed hyperlipidemia due to its impressive effects to lower low-density lipoprotein (ldl) cholesterol levels and increase high-density lipoprotein (hdl) cholesterol levels without any associated major adverse events.tan ey, hartmann g, chen q, pereira a, bradley s, doss g, zhang as, ho jz, braun mp, dean dc, tang w, kumar s. pharmacokinetics, metabolism, and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in rats and rhesus monkeys. drug metab dispos. 2010;38(3):459-473.kumar s, tan ey, hartmann g, biddle z, bergman aj, dru j, ho jz, jones an, staskiewicz sj, braun mp, karanam b, dean dc, gendrano in, graves mw, wagner ja, krishna r. metabolism and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in humans. drug metab dispos. 2010;38(3):474-483
- Enzyme inhibitor This cholesteryl ester transfer protein (or CETP) inhibitor (FW = 637.51 g/mol; CAS 875446-37-0), also known by the code name MK-0859, reduces plasma cholesterol concentrations, thereby increasing serum highdensity lipoprotein levels and preventing cardiovascular disease associated with hypercholesteremia. X-ray crystal studies suggest that CETP is a crescent-shaped protein with a 6-nm hydrophobic tunnel traversing its core Both of the tunnel’s two entrances face the protein’s concave surface, which most likely is the interaction platform for binding HDL particles and engaging its lipoprotein constituents. CETP mediates the reciprocal transfer of neutral lipids (e.g., cholesteryl esters and triglycerides) and phospholipids between different lipoprotein fractions in blood plasma. Anacetrapib is unlike many CETP inhibitors, which increase systolic blood pressure. Anacetrapib also exhibits a low-to-moderate degree of absorption after oral dosing and majority of the absorbed dose is eliminated via oxidation to a series of hydroxylated metabolites that undergo conjugation with glucuronate before excretion into bile. Unlike torcetrapib, another CETP inhibitor, anacetrapib does not elevate blood pressure, alter electrolytes, or cause any significant side effects.
-
in vivo
Hamsters are given Anacetrapib for 7 days before injection of [3H]cholesterol-labeled macrophages (day 0). Treatment with Anacetrapib leads to significant increases in HDL-C levels at day 0. At day 3, [3H]cholesterol radioactivity in the HDL fraction is significantly increased from control values for Anacetrapib[1]. Anacetrapib (ANA) treatment modestly elevates serum total serum cholesterol levels ~10% (p<0.05) and increases serum LDL-C by 26% (p<0.05) as compared to vehicle control[2]. After an intravenous dose of 0.5 mg/kg, the mean values for systemic plasma clearance, steady-state volume of distribution, and terminal half-life are 2.3 mL/min/kg, 1.1 L/kg, and 12 h, respectively. After oral dosing at 5 mg/kg, the bioavailability of Anacetrapib is 38%. Exposures (AUC) increases in a less than dose-proportional manner from 23 μM h at 5 mg/kg to 362 μM h at 500 mg/kg. In this dose range, the peak plasma level (Cmax) ranges from 5 to 26 μM and the time to reach peak plasma level (Tmax) ranged from 3 to 4.5 h[3].
- target rhCETP
-
Supplier:
Seasons Biotechnology Co., Ltd.
- Tel:+86-0576-89232655<br/>+86-13566878689
- Email:info@seasonsbio.com
- Country:China
- ProdList:47
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
Zibo Hangyu Biotechnology Development Co., Ltd
- Tel:+86-0533-2185556<br/>+8615965530500
- Email:nickzhang@hangyubiotech.com
- Country:China
- ProdList:10983
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shanghai Yingrui Biopharma Co., Ltd.
- Tel:+86-21-33585366 - 03@
- Email:sales03@shyrchem.com
- Country:CHINA
- ProdList:738
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Biochempartner
- Tel:0086-13720134139
- Email:candy@biochempartner.com
- Country:CHINA
- ProdList:965
- Advantage:58
- Anacetrapib
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Inhibitors
- Heterocycles
- Chiral Reagents
- Aromatics
- CETP inhibitor
- Inhibitor
- 信号转导通路激酶抑制剂
- 抑制剂
- 药靶配体
- 合成有机化合物配体
- 医药原料
- 日用化学品
- 活性小分子库
- 原料药
- 细胞生物学试剂
- 代谢
- 安塞曲匹
- 康宝泰
- 小分子抑制剂
- 小分子抑制剂,天然产物
- 脂质代谢紊乱
- 家族高胆固醇血症
- 冠状动脉疾病
- 高胆固醇血症
- 胆固醇脂转移蛋白阻滞剂
- 其他科研原料药
- C30H25F10NO3
- 安塞曲匹,10 MM DMSO 溶液
- ANACETRAPIB,胆固醇脂转移蛋白阻滞剂
- (4S,5R)-5-(3,5-双(三氟甲基)苯基)-3-((4'-氟-5'-异丙基-2'-甲氧基-4-(三氟甲基)-[1,1' -联苯]-2-基)甲基)-4-甲基噁唑烷-2-酮
- 安塞曲比,胆固醇脂转移蛋白阻滞剂
- CETP抑制剂(ANACETRAPIB)
- 安赛曲匹
- 安塞曲匹ANACETRAPIB(WITH3INTS.)
- 安塞曲贝
- 安塞曲比
- 安塞曲匹/胆固醇脂转移蛋白阻滞剂
- 安塞曲匹
- 胆固醇脂转移蛋白阻滞剂
- (4S,5R)-5-[3,5双(三氟甲基)苯]-3-({2-[4-氟-2-甲氧基-5-异丙基苯]-5-(三氟甲基)苯}甲基)-4-甲基-1,3-噁唑烷-2-酮
- (4S,5R)-5-[3,5-双(三氟甲基)苯基]-3-({2-[4-氟-2-甲氧基-5-(丙-2-基)苯基]-5-(三氟甲基)苯基}甲基)-4-甲基-1,3-恶唑烷-2-酮
- 胆固醇脂转移蛋白阻滞剂/安塞曲匹
- 胆固醇脂转移蛋白阻滞剂杂质
- 安塞曲匹API
- 875446-37-0
- Anacetrapib, 10 mM in DMSO
- (4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-3-[[2-(4-fluoro-5-isopropyl-2-methoxy-phenyl)-5-(trifluoromethyl)phenyl]methyl]-4-methyl-oxazolidin-2-one
- TIANFUCHEM-- Anacetrapib
- Anacetrapib 13C6
- (4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-3-{[4'-fluoro-2'-...
- Anacetrapib USP/EP/BP
- MK-0859; MK 0859; MK0859
- CS-66
- MK0859; MK-0859; MK 0859; ANACETRAPIB
- (4S,5R)-5-[3,5-Bis(trifluoromethyl)phenyl]-3-({2-[4-fluoro-2-methoxy-5-(propan-2-yl)phenyl]-5-