ChemicalBook > CAS DataBase List > Morphine Sulfate
Morphine Sulfate
Morphine Sulfate
- CAS No.6211-15-0
- Chemical Name:Morphine Sulfate
- CBNumber:CB6140018
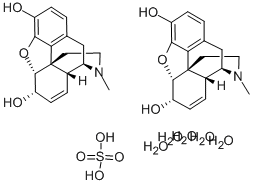
- Molecular Formula:C34H50N2O15S
- Formula Weight:758.83
- MOL File:6211-15-0.mol
Morphine Sulfate Property
- Melting point >240°C (dec.)
- storage temp. Controlled Substance, -20°C Freezer
- solubility ethanol: 1.8 mg/mL
- form A crystalline solid
- BCS Class 3/1
- NCI Dictionary of Cancer Terms morphine sulfate
- FDA UNII X3P646A2J0
- NCI Drug Dictionary morphine sulfate
- UNSPSC Code 41116107
- NACRES NA.24
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H336
- Precautionary statements P261-P264-P270-P271-P301+P312-P304+P340+P312
Morphine Sulfate Chemical Properties,Usage,Production
- Description Morphine (sulfate hydrate) (Item No. 25955) is an analytical reference standard categorized as an opioid. It is a natural phenanthrene commonly used for its analgesic properties but has a high potential for addiction. Morphine is regulated as a Schedule II compound in the United States. This product is intended for research and forensic applications.
- Chemical Properties White Solid
- Originator Avinza,Aetna Inc.
- Uses Principle alkaloid of opium. This is a controlled substance (opiate). Analgesic (narcotic).
- Uses Principal alkaloid of opium. This is a controlled substance (opiate). Analgesic (narcotic)
- Definition ChEBI: Morphine sulfate pentahydrate is an alkaloid sulfate salt and a hydrate. It contains a morphine.
-
Manufacturing Process
Morphin was extracted from the plant vegetable (the poppy) by the mixture of
water and methanol or the aqueous solution of potassium pyrosulfate. The
precipitation of the morphin was carried out by addition to the extract the
aqueous solution of sodium carbonate.
Morphin can be obtained from the extract by using the cation exchanger.
Free base of morphin was transformated to the sulfate salt. - brand name Astramorph (AstraZeneca); Avinza (Ligand); Depodur (Skyepharma); Duramorph (Baxter Healthcare); Infumorph (Baxter Healthcare); Kadian (Alpharma); Ms Contin (Purdue Frederick); Oramorph (Xanodyne).
- Therapeutic Function Narcotic analgesic, Sedative
- Acquired resistance The analgesic effect of orally dosed morphine can equal that obtained by parenteral administration, if proper doses are given. When given orally, the initial dose of morphine is usually 60 mg, followed by maintenance doses of 20 to 30 mg every 4 hours. Addiction to clinically used morphine by the oral route generally is not a problem.
- Mechanism of action Morphine sulfate is the analgesic used most often for severe, acute, and chronic pain. Morphine is a μ agonist and is a Schedule II drug. It is available in intramuscular, subcutaneous, oral, rectal, epidural, and intrathecal dosage forms. The epidural and intrathecal preparations are formulated without a preservative.Morphine is three- to six times more potent when given intramuscularly than when given orally. The difference in activity results from extensive first-pass 3-O-glucuronidation of morphine—an inactive metabolite. The half-life of intramuscularly dosed (10 mg) morphine is approximately 3 hours. The dose of morphine, by any dosage route, must be reduced in patients with renal failure and in geriatric and pediatric patients. The enhanced effects of morphine in renal failure is believed to be caused by a buildup of the active 6-glucuronide metabolite, which depends on renal function for elimination.
Morphine Sulfate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(0)Suppliers
6211-15-0, Morphine SulfateRelated Search:
- Intermediates & Fine Chemicals
- Pharmaceuticals
- 2C17H19NO3H2O4S5H2O
- C34H40N2O10S5H2O
- 2C17H19NO35H2OH2O4S
- C34H50N2O15S
- 吗啡硫酸盐五水合物
- 吗啡五水硫酸盐
- 6211-15-0
- Apomorphine Hydrochloride Hemihydrate EP Impurity B
- Codeine Monohydrate Impurity B as Hemisulphate Pentahemihydrate
- Ethylmorphine Hydrochloride Impurity B as Hemisulfate Pentahemihydrate
- Apomorphine impurity B (morphine sulfate hydrate)
- Morphine Impurity 1
- Apomorphine impurity B (morphine sulfate)
- Morphine Hemisulphate Pentahemihydrate
- Morphine sulfate - * narc CRS (Y0000452)
- Morphine Sulfate 20 mg Tablets
- morphine sulfate--dea schedule ii item
- Ospalivina Sulfate Hydrate
- Nepenthe Sulfate Hydrate
- Morphine Sulfate Pentahydrate USP
- Morphina Sulfate Hydrate
- Morphia Sulfate Hydrate
- Aguettant Sulfate Hydrate
- (5a,6a)-7,8-Didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol Sulfate Hydrate
- 4,5-alpha-epoxy-17-methylmorphinan-3,5-alpha-diol sulfate hydrate
- MORPHINESULFATE,USP
- morphine sulfate salt pentahydrate
- Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-methyl- (5.alpha.,6.alpha.)-, sulfate (2:1) (salt), pentahydrate
- MORPHINE HEMI[SULFATE PENTAHYDRATE]
- MORPHINE SULFATE PENTAHYDRATE
- MORPHINE SULFATE HYDRATE
- MORPHINE SULFATE
- te(2:1)(salt),pentahdrate
- 6-diol,7,8-didehydro-4,5-epoxy-17-methyl-(5-alpha,6-alpha)-morphinan-sulfa
- 4,5-alpha-epoxy-17-methylmorphinan-3,5-alpha-diolsulfatehydrate(2:1:5)
- Morphine pentahydrate sulfate salt
- Morphine sulfat
- Morphine for system suitability
- Morphine Sulfate (CII), USP
- Morphine Sulfate CII (500 mg)
- Kadian (tn)
- Depodur (tn)
- Depodur
- D00842
- Avinza (tn)
- Astramor ph (tn)
- Astramor ph