ChemicalBook > CAS DataBase List > Famotidine
Famotidine
Famotidine
- CAS No.76824-35-6
- Chemical Name:Famotidine
- CBNumber:CB5706585
- Molecular Formula:C8H15N7O2S3
- Formula Weight:337.45
- MOL File:76824-35-6.mol
Famotidine Property
- Melting point 163-164°C
- Boiling point 562.7±60.0 °C(Predicted)
- Density 1.5111 (rough estimate)
- vapor pressure <0.0000001 kPa ( 25 °C)
- refractive index 1.7400 (estimate)
- storage temp. 2-8°C
- solubility Very slightly soluble in water, freely soluble in glacial acetic acid, very slightly soluble in anhydrous ethanol, practically insoluble in ethyl acetate. It dissolves in dilute mineral acids
- pka pKa 6.76(H2O t=23.0) (Uncertain)
- form Solid
- color White to Off-White
- biological source synthetic
- Water Solubility 1.1 mg/mL
- BCS Class 3
- Stability Light Sensitive
- InChI InChI=1S/C8H15N7O2S3/c9-6(15-20(12,16)17)1-2-18-3-5-4-19-8(13-5)14-7(10)11/h4H,1-3H2,(H2,9,15)(H2,12,16,17)(H4,10,11,13,14)
- InChIKey XUFQPHANEAPEMJ-UHFFFAOYSA-N
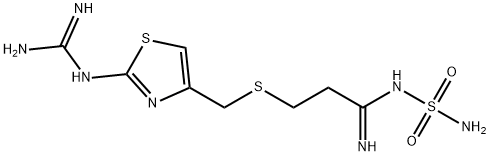
- SMILES C(NS(N)(=O)=O)(=N)CCSCC1=CSC(NC(N)=N)=N1
- CAS DataBase Reference 76824-35-6(CAS DataBase Reference)
- FDA UNII 5QZO15J2Z8
- NCI Drug Dictionary famotidine
- ATC code A02BA03,A02BA53
- EPA Substance Registry System Propanimidamide, 3-[[[2-[(aminoiminomethyl)amino]-4-thiazolyl]methyl]thio]-N-(aminosulfonyl)- (76824-35-6)
- UNSPSC Code 41116107
- NACRES NA.77
Safety
- Hazard Codes :T
- Risk Statements :20/21/22-45-61
- Safety Statements :22-24/25-53-45-36/37/39
- WGK Germany :2
- RTECS :UA2300000
- HS Code :29341000
- Hazardous Substances Data :76824-35-6(Hazardous Substances Data)
- Toxicity :LD50 i.v. in mice: 244.4 mg/kg (Yasufumi)
-
NFPA 704:
0 3 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H303
- Precautionary statements P270-P301+P312-P403-P501c
Famotidine Price
More Price(6)
- Brand: Sigma-Aldrich(India)
- Product number: F6889
- Product name : Famotidine
- Purity:
- Packaging: 500MG
- Price: ₹7436.78
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: PHR1055
- Product name : Famotidine
- Purity: Pharmaceutical Secondary Standard; Certified Reference Material
- Packaging: 1G
- Price: ₹8465.15
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: F6889
- Product name : Famotidine
- Purity:
- Packaging: 1G
- Price: ₹13271.45
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: F6889
- Product name : Famotidine
- Purity:
- Packaging: 5G
- Price: ₹51732.68
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: F0530
- Product name : Famotidine
- Purity:
- Packaging: 5G
- Price: ₹9600
- Updated: 2022/05/26
- Buy: Buy
Famotidine Chemical Properties,Usage,Production
- Description Famotidine (Chemical formula: C8H15N7O2S3; Brand Name: PEPCID) belongs to a histamine H2-receptor antagonist. It appears as a white to pale yellow crystalline compound. Inside the body, its primary activity is inhibiting the gastric secretion process, further reducing the acid concentration and volume of gastric secretion in the stomach. Based on this property, it is used for the treatment and prevention of ulcers occurring in the stomach and intestines. It can also treat diseases such as Zollinger-Ellison syndrome in which the stomach accumulates excess amount of acids. Moreover, it is also applied during the treatment of gastroesophageal reflux disease (GERD) and pathological hypersecretory conditions.
-
Reference
http://www.rxlist.com/pepcid-drug/clinical-pharmacology.htm
https://en.wikipedia.org/wiki/Famotidine - Description Famotidine is a competitive histamine H2-receptor antagonist, and the main pharmacodynamic effect of famotidine is to cause the inhibition of gastric secretion. Famotidine on decomposition releases toxic products such as carbon oxides (CO, CO2), nitrogen oxides (NO, NO2), and sulphur oxides (SO2, SO3). Famotidine is a medication that is available both in prescription and over-the-counter forms. It is used to treat conditions related to the oesophagus, stomach, and intestines. Some specific famotidine is used for the treatment of duodenal ulcers, gastric ulcers (stomach ulcers), gastroesophageal reflux disease (GERD), and pathological hypersecretory conditions that occur when stomach acid is secreted/ produced in very large quantities, an abnormal health condition called ‘Zollinger-Ellison syndrome’.
- Chemical Properties White Powder
- Originator Yamauouchi (Japan)
- Uses For the treatment of peptic ulcer disease (PUD) and gastroesophageal reflux disease (GERD).
- Uses antiinflammatory
- Uses Histamine H2-receptor antagonist. Antiulcerative.
- Uses Use as an H2-antagonist. An anti-ulcer agent
- Uses Contact dermatitis from famotidine, a H2 -receptor agonist, was described in a nurse. In industry, three cases were reported due to intermediates of synthesis, 2- diamino-ethylene-amino-thiazolyl-methylenethiourea-dichloride and 4-chloromethyl-2-guanidinothiazolenitrochloride.
-
Manufacturing Process
60.0 kg of dichloroacetone is dissolved in 550 ml of acetone. After cooling the
solution to -5°C, 55.8 kg of amidinothiourea is added to the solution under
cooling portionwise at one hour intervals in a 10 kg amount of
amidinothiourea. The mixture is stirred continuously for 5 days below 0°C.
The 111.6 kg resultant precipitates of N"-[4-(chloromethyl)-4,5-dihydro-4-
hydroxy-2-thiazolyl]-guanidine hydrochloride are collected, and washed with
50 L of acetone. In 500 ml of water are dissolved 111.6 kg of N"-[4-
(chloromethyl)-4,5-dihydro-4-hydroxy-2-thiazolyl]-guanidine hydrochloride
and 32.9 kg of thiourea. The solution is stirred for one hour at 50°C. N'-[4-
[[(Aminoiminomethyl)thio]methyl]-2-thiazolyl]-guanidine dihydrochloride is
formed in the reaction mixture, and this reaction mixture containing this
compound is directly used for the next process without isolation of the formed
compound.
The reaction mixture obtained is cooled below 10°C, and to the solution are added 45.6 kg of beta-chloropropionitrile and 200 L of isopropanol. A solution of 69.1 kg of sodium hydroxide in 280 L of water is added dropwise to the solution under nitrogen stream followed by stirring for 2 hours at 0°C. The crystals precipitated are collected by filtration, and washed with cold water and dried to provide 91.7 kg of the N"-[4-[[(2-cyanoethyl)thio]methyl]-2- thiazolyl]-guanidine, melting point 125-126.5°C.
In 60 L of anhydrous dimethylformamide is dissolved 34.3 kg of the N"-[4- [[(2-cyanoethyl)thio]methyl]-2-thiazolyl]-guanidine. After adding 60 L of anhydrous methanol to the solution, 61.9 kg of hydrogen chloride gas is passed through the solution below 5°C. After stirring the reaction mixture for 2 days at 0°C, the reaction mixture is poured into a mixture of 350 L of water, 250 kg of potassium carbonate, 30 L of ethyl acetate and ice while stirring below 5°C for 2 hours. The resultant precipitates are collected by filtration. After stirring a mixture of the precipitates and 400 L of water for 0.5 hour at 0°, the resultant precipitates are collected by filtration, washed with 40 L of water and 10 L of cooled acetone respectively, and dried at reduced pressure to provide 30.6 kg of the methyl 3-[[[2-[(diaminomethylene)amino]-4- thiazolyl]methyl]thio]propionimidate showing a melting point of 125.7°C.
In 340 L of methanol is dissolved 88.4 kg of sulfamide under heating, and the solution is cooled to 30°C. To the solution, 114.2 kg of the methyl 3-[[[2- [(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propionimidate are added portionwise three times while stirring at 20-30°C. (The second addition is added 8 hours after the first addition, and the third addition is added 24 hours after the first addition). After stirring the reaction mixture for a further 2 days, the crystals formed are collected by filtration, washed with 200 L of cooled methanol, and air-dried at room temperature to provide 87.5 kg of the 3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]-Nsulfamoylpropionamidine (generic name: famotidine) showing a melting point of 157.6°C. Some of the obtained product is recrystallized from dimethylformamide-water, and is dissolved in an equivalent molar amount of aqueous acetic acid (%). To the solution is added an equivalent molar amount of a dilute sodium hydroxide solution in water to separate crystals showing a melting point of 163-164°C. - brand name Fluxid (Schwarz Pharma); Pepcid (Merck);Amifatidine;Famodil;Pepsidac;GASTER.
- Therapeutic Function Antiulcer
-
General Description
Famotidine is a histamine H2-receptor antagonist, which promotes the healing of erosive esophagitis, gastric and duodenal ulcers since it inhibits the gastric acid secretion in humans.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. - Contact allergens Contact dermatitis in a nurse from famotidine, an H2-receptor agonist, was described. In industry, three cases were reported due to intermediates of the synthesis of 2-diamino-ethylene-amino-thiazolyl-methylenethio urea-dichloride, and 4-chloromethyl-2-guanidinothiaz ole-nitrochloride.
- Biochem/physiol Actions H2 histamine receptor antagonist; anti-ulcer agent
- Clinical Use Famotidine is a histamine H2-antagonist more potent than cimetidine and ranitidine. Administered once or twice daily, it is useful in the treatment of gastric, duodenal and anastomotic ulcers, upper gastrointestinal tract hemorrhage, reflux esophagitis and Zollinger-Ellison syndrome. Like ranitidine, it is lacking in antiandrogenic effects.
-
Synthesis
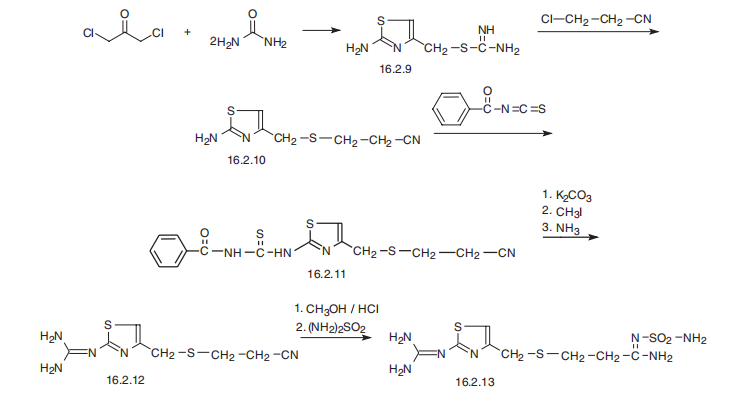
Famotidine, 3-[[[2-[(aminomethyl)amino]-4-thiazolyl] methyl]thio]-
N-(aminosulfonyl)propanimidamide (16.2.13), is synthesized from S-(2-aminothiazol-4-ylmethyl)
isothiourea (16.2.9), which is synthesized by reacting 1,3-dichloroacetone with two
molecules of thiourea, during which a thiazol ring is formed and the chlorine atom is substituted,
giving an intermediate 2-amino-5-chlormethylthiazol. Reacting this with 2-chlorpropionitrile
gives S-(2-aminothiazol-4-yl-methyl)-2-cyanoethane (16.2.10), which in turn is
reacted with benzoylizthiocyanate. The resulting benzoylthiourea derivative (16.2.11) first
undergoes S-methylation by methyliodide and further cleaved by ammonia into 3-[[[2-
(aminomethyl)amino]-4-thiazolyl]-methyl]thio]ethylcyanide (16.2.12). Successive
methanolysis of the nitrile group and subsequent reaction of the resulting iminoether with
sulfonamide gives famotidine (16.2.13).
-
Veterinary Drugs and Treatments
In veterinary medicine, famotidine may be useful for the treatment
and/or prophylaxis
of gastric, abomasal and duodenal ulcers,
uremic gastritis, stress-related or drug-induced erosive gastritis,
esophagitis, duodenal gastric reflux, and esophageal reflux.
Famotidine has fewer drug interactions and activity may persist longer than cimetidine. - Drug interactions Potentially hazardous interactions with other drugs Antifungals: absorption of itraconazole and ketoconazole reduced; concentration of posaconazole possibly reduced - avoid with suspension. Antivirals: concentration of atazanavir reduced - adjust doses of both drugs; concentration of raltegravir possibly increased - avoid; avoid for 12 hours before and 4 hours after rilpivirine. Ciclosporin: possibly increased ciclosporin levels. Cytotoxics: possibly reduced dasatinib concentration - avoid if possible; avoid with erlotinib; possibly reduced absorption of pazopanib - give at least 2 hours before or 10 hours after famotidine; possibly reduced absorption of lapatinib. Ulipristal: contraceptive effect possibly reduced - avoid with high dose ulipristal.
- Metabolism Metabolism of famotidine occurs in the liver, with formation of an inactive metabolite, the sulfoxide. Following oral administration, the mean urinary excretion of famotidine is 65-70% of the absorbed dose, 25-30% as unchanged compound. Renal clearance is 250-450 mL/min, indicating some tubular excretion. A small amount may be excreted as the sulfoxide.
- storage Store at -20°C
Famotidine Preparation Products And Raw materials
Raw materials
Preparation Products
Global(707)Suppliers
- Americas 1 Austria 2 Brazil 2 Canada 8 China 395 Europe 4 France 4 Germany 9 Hungary 2 India 168 Indonesia 1 Japan 5 Jordan 1 Malaysia 1 Nepal 1 Nigeria 1 Philippines 1 Poland 3 Russia 1 Slovenia 1 South Korea 3 Spain 6 Switzerland 3 The Netherlands 2 United Arab Emirates 1 United Kingdom 17 United States 63 Uruguay 1 Global 707
-
Supplier:
Hebei Kangcang new material Technology Co., LTD
- Tel: +8615713292910
- Email:Nancy@kangcang.com.cn
- Country:China
- ProdList:341
- Advantage:58
-
Supplier:
Liaoning Pharmaceutical Innovation Co., Ltd
- Tel: +8616588669988
- Email:sales@pipharma.com.cn
- Country:China
- ProdList:151
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
Anhui Ruihan Technology Co., Ltd
- Tel: +8617756083858
- Email:daisy@anhuiruihan.com
- Country:China
- ProdList:973
- Advantage:58
-
Supplier:
Hangzhou Hyper Chemicals Limited
- Tel:+86-0086-57187702781<br/>+8613675893055
- Email:info@hyper-chem.com
- Country:China
- ProdList:295
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Rixing Chemical CO.,LTD
- Tel:+86-13237129059<br/>-13237129059
- Email:sales@rixingchemi.com
- Country:CHINA
- ProdList:229
- Advantage:55
Related articles
76824-35-6, FamotidineRelated Search:
- Methylchloroisothiazolinone/methylisothiazolinone mixture (MCIT/MIT) Basic Violet 1 Methyl salicylate METSULFURON METHYL N-Aminothiourea Sodium thiosulfate Sulfamide Thioacetamide Tribenuron methyl Sulfanilamide Kresoxim-methyl Methyl acrylate N-Acetylsulfanilyl chloride Methanol Benzothiazole Methylparaben Methyl [3-[[[2-(DiaMinoMethyleneaMino)-4-thiazolyl]Methyl]thio]propionyl]sulfaMide Hydrochloride (FaMotidine IMpurity)
- 76824-35-6
- API's
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Histamine receptor
- Sulfur & Selenium Compounds
- Heterocycles
- Amines
- LODINE
- Other APIs
- 抑制剂
- 药靶配体
- 高端化学
- 生物试剂
- 杂质对照品
- 对照品
- 有机化学
- 精细化工原料
- 化工中间体-化工原料
- 化学试剂
- 消化类
- 化工原料
- 医药、农药及染料中间体
- 标准品 -对照药材
- 医药生物化工
- 有机化工
- 医用原料
- 原料中间体-原料药
- 优势产品
- 生化试剂
- 原料
- 化合物:原料药:兽用
- 原料药
- 医药原料
- 医药中间体
- 神经信号
- 医药原料药
- 药物
- 组胺H2受体拮抗剂
- 抗组胺药
- 小分子抑制剂
- 植物提取物
- C8H15N7O2S3
- C18H15N7O2S3
- FAMOTIDINE,H2拮抗剂
- 法莫替丁/用于系统适用性试验/A1A
- 甲醇中法莫替丁标准溶液
- 法莫替丁(对照品)
- 法莫替丁(注射剂)
- 3-(((2-胍基噻唑-4-基)甲基)硫基)-N-氨基磺酰基丙脒
- 法莫替丁, 组胺H2受体拮抗剂
- 法莫替丁FAMOTIDINE
- 法莫替丁 EP标准品
- 法莫替丁 USP标准品
- 法莫替丁系统适应性 EP标准品
- 法莫替丁粗品
- 法莫替丁-D3-13C
- 法莫替丁价格|法莫替丁生产厂家