ChemicalBook > CAS DataBase List > 1-ACETYLINDOLINE
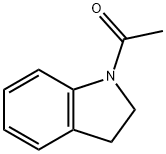
1-ACETYLINDOLINE
1-ACETYLINDOLINE
- CAS No.16078-30-1
- Chemical Name:1-ACETYLINDOLINE
- CBNumber:CB5487514
- Molecular Formula:C10H11NO
- Formula Weight:161.2
- MOL File:16078-30-1.mol
1-ACETYLINDOLINE Property
- Melting point 102-104 °C(lit.)
- Boiling point 125-130 °C(Press: 1 Torr)
- Density 1.144±0.06 g/cm3(Predicted)
- storage temp. Sealed in dry,Room Temperature
- pka 1.13±0.20(Predicted)
- CAS DataBase Reference 16078-30-1(CAS DataBase Reference)
- UNSPSC Code 12352100
- NACRES NA.22
Safety
- Safety Statements :22-24/25
- WGK Germany :3
- RTECS :NM1892500
- HS Code :2933998090
-
NFPA 704:
1 2 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H317
- Precautionary statements P280
1-ACETYLINDOLINE Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 379492
- Product name : 1-Acetylindoline
- Purity: 98%
- Packaging: 5G
- Price: ₹4384.13
- Updated: 2022/06/14
- Buy: Buy
1-ACETYLINDOLINE Chemical Properties,Usage,Production
- Uses 1-(2,3-Dihydroindol-1-yl)ethanone is an intermediate used for preparation of triazolothiadiazepine dioxide derivatives, halo-substituted aromatic amides and [[(phenyl)piperazinyl]alkyl]indolyl]ethanone.
- Uses • ;Reactant for preparation of triazolothiadiazepine dioxide derivatives1• ;Reactant for preparation of halo-substituted aromatic amides2• ;Reactant for preparation of [[(phenyl)piperazinyl]alkyl]indolyl]ethanone and [[[(phenoxyethyl)piperazinyl]alkyl]indolyl]ethanone derivatives as α1-adrenoreceptor antagonists3• ;Reactant for synthesis of naphthalenedione derivatives as antimycobacterial agents4• ;Reactant for regioselective ortho Suzuki-Miyaura coupling reaction5
-
Uses
- Reactant for preparation of triazolothiadiazepine dioxide derivatives
- Reactant for preparation of halo-substituted aromatic amides
- Reactant for preparation of [[(phenyl)piperazinyl]alkyl]indolyl]ethanone and [[[(phenoxyethyl)piperazinyl]alkyl]indolyl]ethanone derivatives as α1-adrenoreceptor antagonists
- Reactant for synthesis of naphthalenedione derivatives as antimycobacterial agents
- Reactant for regioselective ortho Suzuki-Miyaura coupling reaction
-
Synthesis Reference(s)
Tetrahedron, 52, p. 7525, 1996 DOI: 10.1016/0040-4020(96)00266-9
Tetrahedron Letters, 24, p. 561, 1983 DOI: 10.1016/S0040-4039(00)81464-1 - General Description 1-Acetylindoline, a substituted indole, is a tertiary amide having bulky N-groups. Its reduction to the corresponding amine, by reaction with silane in the presence of MoO2Cl2 (catalyst), has been reported.
1-ACETYLINDOLINE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(203)Suppliers
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
SHAANXI WEISHINUO THE NEW MATERIALCO,.LTD
- Tel:18792503214
- Email:Beryl@weishino.com
- Country:CHINA
- ProdList:256
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
SIMAGCHEM CORP
- Tel: +86-13806087780
- Email:sale@simagchem.com
- Country:China
- ProdList:17365
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
16078-30-1, 1-ACETYLINDOLINERelated Search:
- Indoline N-ACETYLINDOLE-3-CARBOXALDEHYDE 1-ACETYL-5-BROMOINDOLINE STRYCHNINE 1-ACETYL-5-AMINO-2,3-DIHYDRO-(1H)-INDOLE STRYCHNINE 10,11-Dimethoxystrychnine BRUCINE SULFATE Strychnine Nitrate 1-ACETYL-6-AMINOINDOLINE STRYCHNINE HYDROCHLORIDE 1-ACETYL-5-BROMO-7-NITROINDOLINE 1-ACETYLINDOLINE 1-ACETYL-5-NITROINDOLINE BRUCINE HYDROCHLORIDE 1-acetylindoline-2,3-dione STRYCHNINE-N-OXIDE 1-ACETYL-6-CHLOROINDOLINE
- Indoles and derivatives
- ACETYLGROUP
- Indoline & Oxindole
- Indoles
- Heterocyclic Building Blocks
- Building Blocks
- Heterocyclic Building Blocks
- Chemical Synthesis
- C10
- Building Blocks
- 化工原料及中间体
- 高端化学
- 医用原料
- 医药原料
- 有机化工原料
- 有机化学
- 化工原料
- 有机砌块
- 杂环砌块
- 吲哚
- 杂环化合物
- Building Blocks
- Heterocyclic Building Blocks
- Indoles
- PERFEMIKER]N-乙酰基吲哚啉,95%
- 1-(2,3-二氢-1H-吲哚-1-基)乙烷-1-酮
- 乙酰基吲哚啉
- N-乙酰基吲哚啉
- 1-已酰基吲哚啉
- 1-乙酰-二氢吲哚
- N-已酰基吲哚啉
- 1-乙酰基吲哚啉
- 16078-30-1
- 1-(indolin-1-yl)ethan-1-one
- Ethanone, 1-(2,3-dihydro-1H-indol-1-yl)-
- 1-Acetyl-2,3-dihydro-1H-indole
- 1H-Indole, 1-acetyl-2,3-dihydro-
- 1-ACETYLINDOLINE 98+%
- N-ACETYL INDOLINE
- 1H-Indole, 1-acetyl-2,3-dihydro- (9CI)
- Acetylindoline,N-
- 1-ACETYLINDOLINE
- 1-ACETYL-2,3-DIHYDROINDOLE
- 1-(2,3-DIHYDRO-INDOL-1-YL)-ETHANONE
- 1-(2,3-DIHYDRO-1H-INDOL-1-YL)ETHAN-1-ONE
- 1-acetyl-indolin
- 1-acetyl-2,3-dihydro-1h-indol