ChemicalBook > CAS DataBase List > IBUFENAC
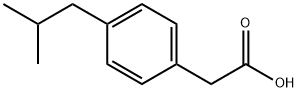
IBUFENAC
IBUFENAC
- CAS No.1553-60-2
- Chemical Name:IBUFENAC
- CBNumber:CB4506742
- Molecular Formula:C12H16O2
- Formula Weight:192.25
- MOL File:1553-60-2.mol
IBUFENAC Property
- Melting point 85-87°C
- Boiling point 288.25°C (rough estimate)
- Density 1.0240 (rough estimate)
- refractive index 1.5100 (estimate)
- storage temp. Sealed in dry,Store in freezer, under -20°C
- solubility Chloroform (Slightly), Methanol (Slightly)
- form Solid
- pka 4.36±0.10(Predicted)
- color White to Off-White
- FDA UNII 5V4WIX44VI
- UNSPSC Code 12352200
- NACRES NA.77
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P305+P351+P338
IBUFENAC Chemical Properties,Usage,Production
- Chemical Properties Crystalline Solid
- Uses Used as an analgesic, anti-inflammatory.
- Definition ChEBI: A monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is replaced by a 4-isobutylphenyl group. Although it was shown to be effective in treatment of rheumatoid arthritis, the clinical use of ibufenac was discontinued due to hepatot xic side-effects.
- Biological Activity ibufenac is a dual cox-1 and -2 inhibitor.cyclooxygenase (cox), also known as prostaglandin-endoperoxide synthase (ptgs), is an enzyme that is responsible for formation of prostanoids, such as thromboxane and prostaglandins.
- in vitro ibufenac was identified as an analog of the nsaid ibuprofen that could inhibit cox-1 and -2 activity with ic50 values of 17.4 and 13.1 μm, respectively [1].
- in vivo in a previous animal study, two new structural analogs, r3 and r4, along with their parent compounds, ibufenac and ibuprofen, were evaluated for their biopharmaceutical properties. aanti-inflammatory activity was evaluated by topically administering drugs to inhibit inflammation induced by using either clove oil or arachidonic acid. results showed that the rank order of activity was ibufenac approximately equal to ibuprofen > r3 approximately equal to r4 [2].
- IC 50 17.4 and 13.1 μm for cox-1 and -2, respectively.
-
References
[1] gülcan, h. o.,nlü, s.,dimoglo, a., et al. marginally designed new profen analogues have the potential to inhibit cyclooxygenase enzymes. arch.pharm.chem.life sci. 348, 55-61 (2015).
[2] rao cs, schoenwald rd, barfknecht cf, laban sl. biopharmaceutical evaluation of ibufenac, ibuprofen, and their hydroxyethoxy analogs in the rabbit eye. j pharmacokinet biopharm. 1992 aug;20(4):357-88.
[3] t. m. chalmers.
IBUFENAC Preparation Products And Raw materials
Raw materials
Preparation Products
Global(79)Suppliers
-
Supplier:
Changzhou Ansciep Chemical Co., Ltd.
- Tel:+86 519 86305871
- Email:sales@ansciepchem.com
- Country:CHINA
- ProdList:4241
- Advantage:58
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Win-Win chemical CO., Limited
- Tel:+86-0086-577-64498589<br/>+86-15355981851
- Email:sales@win-winchemical.com
- Country:China
- ProdList:14442
- Advantage:58
-
Supplier:
Baoji Guokang Bio-Technology Co., Ltd.
- Tel:0917-3909592<br/>13892490616
- Email:gksales1@gk-bio.com
- Country:China
- ProdList:9304
- Advantage:58
-
Supplier:
Changzhou Anxuan Chemical Co., Ltd
- Tel:+86-0519-89882563<br/>+86-18961279162
- Email:andy@anxuanchem.com
- Country:China
- ProdList:4327
- Advantage:58
-
Supplier:
LEAP CHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:24727
- Advantage:58
-
Supplier:
Aceschem Inc.
- Tel:+1-817863-6948<br/>+1-(817)863-6948
- Email:sales@aceschem.com
- Country:United States
- ProdList:19632
- Advantage:58
-
Supplier:
Wuhan Topule Biopharmaceutical Co., Ltd
- Tel: +8618327326525
- Email:masar@topule.com
- Country:China
- ProdList:8467
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:52924
- Advantage:58
1553-60-2, IBUFENACRelated Search:
- methyl 2-(4-isobutylphenyl)acetate Ibufenac Acyl-β-D-Glucuronide ethyl 4-isobutylphenylacetate Loxoprofen IBUPROFEN SODIUM SALT (S)-(+)-Ibuprofen IBUFENAC Ibuprofen Lobuprofen Butibufen Metoxibutropate Ibuprofen Acyl-b-D-glucuronide (R)-(-)-IBUPROFEN METHYL 2-(4-ISOBUTYLPHENYL)PROPANOATE IBUPROFENPICONOL IBUPROFEN, RS, [CARBOXYL-14C] Isoprofen Mexoprofen
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Inhibitors
- 原料药
- 小分子
- 芳烃
- 布洛芬23
- 4-异丁基苯基乙酸
- 异丁苯乙酸
- 对异丁苯乙酸(CAS号:1553-60-2)
- 异丁芬酸
- 异丁布芬酸
- 去甲布洛芬
- 对异丁苯乙酸
- 布芬酸
- 异丁芬酸 (布芬酸、对异丁苯乙酸、去甲布洛芬、异丁布芬酸、对异丁基苯乙酸)
- 对异丁基苯乙酸
- 1553-60-2
- 2-(4-Isobutylphenyl)acetic acid
- 2-(4-Isobutylphenyl)
- Ibufenac [(p-isobutylphenyl)aceticacid]
- Benzeneacetic acid,4-(2-methylpropyl)-
- NSC 99976
- 4-Isobutoxyphenylacetic Acid
- 4-(2-Methylpropyl)phenylacetic Acid
- 2-[4-(2-Methylpropyl)phenyl]acetic acid
- 4-(2-Methylpropyl)benzeneaceticAcid,Dytransin,Ibunac
- p-isobutyl-alpha-toluicacid
- medirex
- kyselinap-isobutylfenyloctova
- isodilan
- ibunac
- ibufenac
- dytransin
- 4-(2-methylpropyl)benzeneaceticacid
- 4-(2-methylpropyl)-benzeneaceticaci
- (p-isobutylphenyl)aceticacid
- (p-isobutylphenyl)-aceticaci