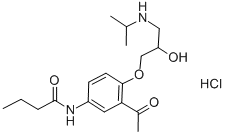
ChemicalBook > CAS DataBase List > Acebutolol hydrochloride
Acebutolol hydrochloride
Acebutolol hydrochloride
- CAS No.34381-68-5
- Chemical Name:Acebutolol hydrochloride
- CBNumber:CB3353130
- Molecular Formula:C18H29ClN2O4
- Formula Weight:372.89
- MOL File:34381-68-5.mol
Acebutolol hydrochloride Property
- Melting point 141-1430C
- storage temp. 2-8°C
- solubility Freely soluble in water and in ethanol (96 per cent), very slightly soluble in acetone and in methylene chloride.
- form Solid
- color White to Pale Yellow
- InChIKey KTUFKADDDORSSI-UHFFFAOYSA-N
- CAS DataBase Reference 34381-68-5(CAS DataBase Reference)
- FDA UNII B025Y34C54
Safety
- Hazard Codes :Xn
- Risk Statements :20/21/22
- Safety Statements :36
- WGK Germany :3
- RTECS :ES5235000
- HS Code :2924296000
- Hazardous Substances Data :34381-68-5(Hazardous Substances Data)
- Toxicity :LD50 orl-rat: 6620 mg/kg OYYAA2 20,883,80
-
NFPA 704:
0 3 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H312+H332
- Precautionary statements P261-P271-P280-P302+P352+P312-P304+P340+P312-P362+P364
Acebutolol hydrochloride Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: PHR1907
- Product name : Acebutolol hydrochloride
- Purity: Pharmaceutical Secondary Standard; Certified Reference Material
- Packaging: 250MG
- Price: ₹23739.23
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: A3669
- Product name : Acebutolol hydrochloride
- Purity: analytical standard
- Packaging: 1G
- Price: ₹4330
- Updated: 2022/06/14
- Buy: Buy
Acebutolol hydrochloride Chemical Properties,Usage,Production
- Chemical Properties Off-White Powder
- Originator Sectral ,May and Baker ,UK ,1975
- Uses 5-alpha-reductase inhibitor
- Uses Cardioselective β-adrenergic blocker. Antihypertensive; antianginal; antiarrhythmic (class II).
- Uses Cardioselective ?adrenergic blocker. Antihypertensive; antianginal; antiarrhythmic (class II)
- Definition ChEBI: The hydrochloride salt of acebutolol, prepared using equimolar amounts of acebutolol and hydrogen chloride.
-
Manufacturing Process
Crude 5'-butyramido-2'-(2,3-epoxypropoxy)acetophenone (16 g),
isopropylamine (20 g) and ethanol (100 ml) were heated together under
reflux for 4 hours. The reaction mixture was concentrated under reduced
pressure and the residual oil was dissolved in N hydrochloric acid. The acid
solution was extracted with ethyl acetate, the ethyl acetate layers being
discarded. The acidic solution was brought to pH 11 with 2 N aqueous sodium
hydroxide solution and then extracted with chloroform. The dried chloroform
extracts were concentrated under reduced pressure to give an oil which was
crystallized from a mixture of ethanol and diethyl ether to give 5'-butyramido-
2'-(2-hydroxy-3-isopropylaminopropoxy)acetophenone (3 g), MP 119-123°C.
Crude 5'-butyramido-2'-(2,3-epoxypropoxy)acetophenone used as starting material was prepared as follows: p-butyramidophenol (58 g; prepared according to Fierz-David and Kuster, Helv. Chim. Acta 1939,2282), acetyl chloride (25.4 g) and benzene (500 ml) were heated together under reflux until a solution formed (12 hours). This solution was cooled and treated with water. The benzene layer was separated and the aqueous layer was again extracted with benzene.
The combined benzene extracts were dried and evaporated to dryness under reduced pressure to give p-butyramidophenyl acetate (38 g) as an off-white solid, MP 102-103°C. A mixture of p-butyramidophenyl acetate (38 g), aluminum chloride (80 g) and 1,1,2,2-tetrachloroethane (250 ml) was heated at 140°C for 3 hours. The reaction mixture was cooled and treated with iced water. The tetrachloroethane layer was separated and the aqueous layer was extracted with chloroform. The combined organic layers were extracted with 2 N aqueous sodium hydroxide and the alkaline solution was acidified to pH 5 with concentrated hydrochloric acid. The acidified solution was extracted with chloroform and the chloroform extract was dried and concentrated under reduced pressure to give 5'-butyramido-2'-hydroxyacetophenone (15.6 g), MP 114-117°C. A solution of 5'-butyramido-2'-hydroxyacetophenone (15.6 g) in ethanol (100 ml) was added to an ethanolic solution of sodium ethoxide which was prepared from sodium (1.62 g) and ethanol (100 ml). The resulting solution was evaporated to dryness under reduced pressure and dimethylformamide (100 ml) was added to the solid residue. Approximately 10 ml of dimethylformamide was removed by distillation under reduced pressure. Epichlorohydrin (25 ml) was added and the solution was heated at 100°C for 4 hours. The solution was concentrated under reduced pressure to give a residual oil which was treated with water to give a solid. The solid was dissolved in ethanol and the resulting solution was treated with charcoal, filtered and concentrated under reduced pressure to give crude 5'-butyramido- 2'-(2,3-epoxypropoxy)acetophenone (16 g), MP 110-116°C.
The crude compound may be purified by recrystallization from ethyl acetate, after treatment with decolorizing charcoal, to give pure 5'-butyramido-2'-(2,3- epoxypropoxy)acetophenone, MP 136-138°C. - brand name Sectral (Dr. Reddy’s).
- Therapeutic Function beta-Adrenergic blocker
- Safety Profile Poison by ingestion, subcutaneous,intravenous, and intraperitoneal routes. An experimentalteratogen. Other experimental reproductive effects. Whenheated to decomposition it emits toxic fumes of NOx andHCl.
Acebutolol hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
Global(222)Suppliers
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
Standardpharm Co. Ltd.
- Tel:86-714-3992388
- Email:overseasales1@yongstandards.com
- Country:United States
- ProdList:14332
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
-
Supplier:
Dideu Industries Group Limited
- Tel:+86-29-89586680<br/>+86-15129568250
- Email:1026@dideu.com
- Country:China
- ProdList:22854
- Advantage:58
34381-68-5, Acebutolol hydrochlorideRelated Search:
- N1-(3-ACETYL-4-HYDROXYPHENYL)ACETAMIDE 3'-Acetamidoacetophenone Acebutolol hydrochloride 2-Acetyl-4-butyramidophenol Practolol 3-AMINO-1-PHENOXY-2-PROPANOL HYDROCHLORIDE, 98 Acebutolol CHEMBRDG-BB 5482082 CHEMBRDG-BB 9070438 1-METHYLAMINO-3-PHENOXY-PROPAN-2-OL AKOS BBS-00007965 1-AMINO-3-PHENOXY-PROPAN-2-OL 3-O-TOLYLOXY-PROPYLAMINE Ethanone, 1-(5-amino-2-methoxyphenyl)- (9CI) METHYL-(3-O-TOLYLOXY-PROPYL)-AMINE CHEMBRDG-BB 5623290 N-(4-propoxyphenyl)acetamide 5'-Amino-2'-hydroxyacetophenone Acebutolol Hcl
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Aromatics
- AVODART
- 抑制剂
- 药靶配体
- 标准品
- 化工原料
- 小分子抑制剂
- AA to AL
- Analytical Chromatography Product Catalog
- Alphabetic
- Analytical Standards
- C18H28N2O4ClH
- C18H28N2O4HCl
- 盐酸醋丁洛尔,10 MM DMSO 溶液
- PERFEMIKER]盐酸醋丁洛尔,98%
- 盐酸醋丁洛尔 USP标准品
- 盐酸醋丁洛尔 EP标准品
- L醋丁洛尔杂质
- 醋丁洛尔盐酸盐
- N-(3-乙酰基-4-[2-羟基-3-(异丙胺基)丙氧基]苯基)丁酰胺
- 盐酸醋丁洛尔溶液, 100PPM
- 醋丁洛尔盐酸盐杂质
- N-[3-乙酰基-4-[2-羟基-3-[(异丙基)氨基]丙氧基]苯基]丁酰胺盐酸盐
- 盐酸醋丁洛尔
- 34381-68-5
- Acebutolol hydrochloride, 10 mM in DMSO
- Acebuprolol hydrochloride
- Acebutolol hydrochloride 10.0 μg/ml Methanol
- H.?O-4,6-dideoxy-4-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino]-α-d-glucopyranosyl-(1→4)-O-6-deoxy-α-d-glucopyranosyl-(1→4)-d-glucopyranose.
- Acetbutolol hydrochloride
- Acebutolol Hydrochloride (1000601)
- Acebutolol Hydrochloride (A0040000)
- Acebutolol hydrochloride USP/EP/BP
- Acebutolol hydrochloride CRS
- N-[3-acetyl-4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]butanamide hydrochloride
- IL-17803A
- Acetanol
- Acecor
- ACEBUTOLOL HYDROCHLORIDE, USP STANDARD
- ACEBUTOLOL HYDROCHLORIDE, IMPURITY IN-[3-ACETYL-4-[(2RS)-3-(ETHYLAMINO)-2-HYDROXYPROPOXY]PHENYL)BUTANAMIDE EP STANDARD
- ACEBUTOLOL HYDROCHLORIDE, MM(CRM STANDARD)
- ACEBUTOLOL HYDROCHLORIDE, IMPURITY CN-(3-ACETYL-4-HYDROXYPHENYL)BUTANAMIDE EP STANDARD
- ACEBUTOLOL HYDROCHLORIDE, EP STANDARD
- (±)-N-[3-acetyl-4-[2-hydroxy-3-[(isopropyl)amino]propoxy]phenyl]butyramide monohydrochloride
- m&b17,803a
- dl-1-(2-acetyl-4-butyramidophenoxy)-2-hydroxy-3-isopropylaminopropanehydroch
- acetobutololhydrochloride
- 3’-acetyl-4’-(2-hydroxy-3-(isopropylamino)propoxy)-butyranilidmonohydrochl
- 3’-acetyl-4’-(2-hydroxy-3-(isopropylamino)propoxy)butyranilidehydrochloride
- Neptall
- M and B 17803A
- DL-1-(2-Acetyl-4-butyramidophenoxy)-2-hydroxy-3-isopropylaminopropane hydrochloride
- DL-1-(2-Acetyl-4-butyramido)-3-(isopropylamino)propan-2-ol hydrochloride
- Butyranilide, 3'-acetyl-4'-[2-hydroxy-3-(isopropylamino)propoxy]-, monohydrochloride, (+-)- (8CI)
- Butanamide, N-3-acetyl-4-2-hydroxy-3-(1-methylethyl)aminopropoxyphenyl-, monohydrochloride
- Sectra