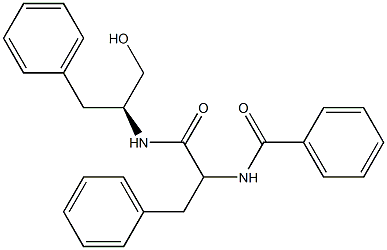
TMC-58B
- CAS No.58115-31-4
- Chemical Name:TMC-58B
- CBNumber:CB32300396
- Molecular Formula:C25H26N2O3
- Formula Weight:402.49
- MOL File:58115-31-4.mol
- Boiling point 723.6±60.0 °C(Predicted)
- Density 1.191±0.06 g/cm3(Predicted)
- pka 13.31±0.46(Predicted)
- form Solid
- color White to off-white
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
TMC-58B Chemical Properties,Usage,Production
- Description One of two new amide-type alkaloids recently isolated from the seeds of Piper aurantiacurn, the structure of this base has been elucidated from chemical degradation and spectroscopic investigations and confirmed by synthesis, the latter also establishing the absolute configuration.
- Uses Aurantiamide is a non-covalent, orally active, blood-brain-permeable GRPR selective antagonist with anti-inflammatory and neuroprotective effects. Aurantiamide reduces inflammation and oxidative stress in renal tissue by inhibiting GRPR-mediated renal necrosis pathways (such as RIPK3/MLKL signaling) and NF-κB inflammatory pathways, exerting anti-acute kidney injury and endothelial function activities. Aurantiamide also inhibits the M1 polarization of microglia and inhibits NLRP3 activation, thereby improving AD mouse models. Aurantiamide has in vivo inhibitory efficacy in acute kidney injury models such as ischemia/reperfusion, sepsis, and hypertension models[1][2][3][4][5].
-
in vivo
Aurantiamide (2.5, 5, 10 mg/kg; oral gavage; 3 times, 24 hours apart) improves cognitive impairment in C57BL/6 mice after ischemia/reperfusion (I/R) and cecal ligation and puncture (CLP) in a dose-dependent manner, and inhibits microglial polarization and NLRP3 activation[2].
Aurantiamide (0.5 mg/kg; intraperitoneal injection; once a day, 5 days a week; 4 weeks) significantly reduces mean arterial blood pressure, improves endothelium-dependent vasodilation, upregulates aortic endothelial nitric oxide synthase (eNOS) protein expression and promotes nitric oxide (NO) production in the two-kidney-one-clip (2K-1C) renovascular hypertension model in Sprague-Dawley rats[4].
The metabolic characteristics of Aurantiamide (0.1 mg/kg; oral gavage; single dose) and Aurantiamide acetate (HY-N2905) (0.2 mg/kg; oral gavage; single dose) in rats shows that they have the characteristics of rapid diffusion, wide distribution, and can pass through the blood-brain barrier, with a peak time of 0.5 h. In addition, the decline rate of aurantiamide acetate is faster than that of aurantiamide[5].Animal Model: Male C57BL/6 mice (6-8 weeks old, 20-22 g) + cisplatin-induced, I/R, or CLP-induced acute kidney injury model[1] Dosage: 2.5, 5, 10 mg/kg (dissolved in 0.5% carboxymethylcellulose + 0.1% Tween 80) Administration: Oral gavage, three times before the surgery, with a 24 h interval between each administration Result: Renal function : Reduced serum creatinine and BUN levels by 30-45% compared to model controls, with the 10 mg/kg dose showing the most pronounced effect.
Histopathology : PAS staining revealed decreased tubular dilation, glycogen deposition, and interstitial fibrosis; immunofluorescence showed reduced KIM1 (renal injury marker) and F4/80+ macrophage infiltration in renal tissues.
Protein expression : Western blot demonstrated dose-dependent inhibition of p-RIPK3, p-MLKL, and p-P65 (NF-κB) in renal lysates, with corresponding reduction in pro-inflammatory cytokines (IL-6, TNF-α) by qPCR.Animal Model: Male Sprague-Dawley rats (8 weeks old, 230-250 g) + two-kidney one-clip (2K-1C) renovascular hypertension model[4] Dosage: 0.5 mg/kg (dissolved in DMSO, final concentration 0.1%) Administration: Intraperitoneal injection, once daily for 5 days/week, total 4 weeks Result: Blood pressure : Reduced mean arterial pressure (MAP) by 20-25% compared to hypertensive controls, with significant improvement in endothelium-dependent relaxation to acetylcholine (ACh) and reduced constriction to phenylephrine (Phe).
Vascular function : Organ bath assays showed enhanced ACh-induced vasodilation and attenuated Phe-induced vasoconstriction in aortic rings, correlated with increased eNOS protein expression (1.5-fold by Western blot) and NO production (measured as nitrite/nitrate levels).
Red blood cell deformability : Ektacytometry revealed increased erythrocyte deformability (Elmax) in treated rats, indicating improved blood fluidity and microvascular flow. - References Banerji, Das,Ind. 1. Chern., 13, 1234 (1975)
-
Supplier:
Chengdu Biopurify Phytochemicals Ltd.
- Tel:+86-28-82633860;<br/>+8618080483897
- Email:sales@biopurify.com
- Country:China
- ProdList:3772
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Labnetwork lnc.
- Tel:+86-27-50766799<br/>+8618062016861
- Email:contact@labnetwork.com
- Country:China
- ProdList:19987
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:
- Email:support@targetmol.com
- Country:United States
- ProdList:38630
- Advantage:58
-
Supplier:
Mensura Group Limited
- Tel: +8618109034517
- Email:1058503172@qq.com
- Country:China
- ProdList:2900
- Advantage:58
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: +8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
-
Supplier:
DONBOO AMINO ACID COMPANY
- Tel: +8618051385538
- Email:donboo@donboo.com
- Country:China
- ProdList:9363
- Advantage:58
- Supplier: Chembest Research Laboratories Limited
- Tel:+86-21-20908456
- Email:sales@BioChemBest.com
- Country:China
- ProdList:6005
- Advantage:61
- Supplier: BioBioPha Co., Ltd.
- Tel:0871-65217109<br/>13211707573;
- Email:y.liu@mail.biobiopha.com
- Country:China
- ProdList:5653
- Advantage:65
- 生物碱类
- 抑制剂
- 对照品
- 标准品 -对照药材
- 生物碱
- 中药对照品
- 对照品
- 植物提取物
- 标准品
- 化合物AURANTIAMIDE,10 MM DMSO 溶液
- 橙黄胡椒酰胺/生物碱类(ALKALOIDS)
- N-((S)-1-(((S)-1-羟基-3-苯基丙-2-基)氨基)-1-氧代-3-苯基丙-2-基)苯甲酰胺
- 化合物AURANTIAMIDE
- 橙黄胡椒酰胺标准品
- 橙黄胡椒酰胺对照品
- 橙黄胡椒酰胺乙酸酯
- 橙黄胡椒酰胺
- 58115-31-4
- Aurantiamide, 10 mM in DMSO
- N-((S)-1-(((S)-1-Hydroxy-3-phenylpropan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)benzamide
- Inhibitor,Aurantiamide,antiplatelet,antitumor,anti-inflammatory,antioxidant,inhibit
- Benzenepropanamide, α-(benzoylamino)-N-[(1S)-1-(hydroxymethyl)-2-phenylethyl]-, (αS)-
- Benzenepropanamide,R-(benzoylamino)-N- [(1S)-1-(hydroxymethyl)-2-phenylethyl]-,(RS)-
- TMC-58B
- Aurantiamide
- (S)-α-(Benzoylamino)-N-[(S)-α-(hydroxymethyl)phenethyl]benzenepropanamide
- (S)-α-(Benzoylamino)-N-[(S)-1-(hydroxymethyl)-2-phenylethyl]benzenepropanamide