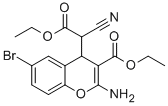
HA14-1
- CAS No.65673-63-4
- Chemical Name:HA14-1
- CBNumber:CB2170185
- Molecular Formula:C17H17BrN2O5
- Formula Weight:409.23
- MOL File:65673-63-4.mol
- Melting point 105 °C
- Density 1.480
- storage temp. Keep in dark place,Sealed in dry,Store in freezer, under -20°C
- solubility DMSO: 26 mg/mL
- form powder
- color White to light yellow
- CAS DataBase Reference 65673-63-4
- UNSPSC Code 12352200
- NACRES NA.77
- Safety Statements :22-24/25
- WGK Germany :3
- HS Code :29322090
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302+H312+H332-H315-H319-H335
- Precautionary statements P280
HA14-1 Chemical Properties,Usage,Production
- Uses HA14-1 is a cell-permeable, nonpeptidic Bcl-2 inhibitor.
- Definition ChEBI: 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-1-benzopyran-3-carboxylic acid ethyl ester is a 1-benzopyran.
- Biological Activity Cell-permeable inhibitor of Bcl-2 protein (IC 50 ~ 9 μ M); acts by binding to the surface pocket. Disrupts Bax/Bcl-2 interaction and induces apoptosis of tumor cells.
- Biochem/physiol Actions HA 14-1 is a nonpeptide apoptosis inducer; Bcl-2 antagonist.
-
in vivo
Swiss nude mice are challenged with BeGBM cells (104 injected s.c.). HA14-1 (400 nmol) in 100 μL free RPMI 1640-50% DMSO, or the carrier alone, is given at the site of injection once weekly from day 2 following cell injection. HA14-1 treatment does not have any significant effect on the growth of glioblastoma tumors in immunodeficient mice as monitored twice weekly by measuring the volume of the expanding tumors. Moreover, no gross organ toxicity or weight loss can be detected in mice receiving such treatment. To analyze whether HA14-1 treatment might enhance the efficiency of another antitumoral treatment, Swiss nude mice challenged with human glioblastoma multiforme cells are also given i.p. low doses of Etoposide (2.5 mg/kg in 200 μL of 0.9% NaCl 5 days a week from day 2 following cell injection) together with HA14-1 or mock treatment. Whereas Etoposide treatment is insufficient by itself to restrain the growth of glioblastoma cells, its combination with HA14-1 leads to a significant restrain on tumor growth as judged by the ability of the combined treatment to increase the doubling time of the tumor volume[3].
- IC 50 Bcl-2: 9 μM (IC50); Bcl-xL
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
SHANGHAI T&W PHARMACEUTICAL CO., LTD.
- Tel:+86-021-61551413<br/>+8618813727289
- Email:contact@trustwe.com
- Country:China
- ProdList:5738
- Advantage:58
-
Supplier:
Tianjin Xinshengjiahe Science & Technology Development Co,.Ltd
- Tel:+86-86-22-87899925<br/>+86-8618522618860
- Email:18522618860@163.com
- Country:China
- ProdList:694
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Dideu Industries Group Limited
- Tel:+86-29-89586680<br/>+86-15129568250
- Email:1026@dideu.com
- Country:China
- ProdList:22854
- Advantage:58
-
Supplier:
Zhejiang J&C Biological Technology Co.,Limited
- Tel:+1-2135480471<br/>+1-2135480471
- Email:sales@sarms4muscle.com
- Country:China
- ProdList:10473
- Advantage:58
-
Supplier:
InvivoChem
- Tel:+1-708-310-1919<br/>+1-13798911105
- Email:sales@invivochem.cn
- Country:United States
- ProdList:6391
- Advantage:58
-
Supplier:
LEAP CHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:24727
- Advantage:58
- 3-(5-BROMO-2-METHOXYPHENYL)PROPANOIC ACID ETHYL 2-CYANOVALERATE 3-(2-METHOXYPHENYL)PROPIONIC ACID 3-Phenylglutaric acid 3-(3-Bromophenyl)propionic acid Ethyl 2-cyanopropanoate METHYL 3-(2-HYDROXYPHENYL)PROPIONATE 3-(3-BROMOPHENYL)PROPIONITRILE 3-(3-Bromophenyl)-2-methylprop-1-ene HA14-1 ETHYL 2-CYANOBUTANOATE 3-(2-HYDROXY-PHENYL)-PROPIONIC ACID ETHYL ESTER ethyl 2-cyano-3-phenyl-propanoate 3-(3-BROMO-PHENYL)-PROPAN-1-OL 2-CYANO-3-PHENYLPROPIONIC ACID 3-(5-bromo-2-methoxy-phenyl)propionitrile 2-Cyano-pentanedioic acid diethyl ester 3-(2-METHOXY-PHENYL)-PROPIONIC ACID ETHYL ESTER
- Inhibitor
- Caspases/Apoptosis
- Inhibitors
- 抑制剂
- 药靶配体
- 标准品
- 小分子抑制剂
- 小分子抑制剂,天然产物
- 细胞凋亡
- Cell Signaling and Neuroscience
- Apoptosis Inducers
- Cell Biology
- BioChemical
- Apoptosis and Cell Cycle
- C17H17BrN2O5
- 化合物HA14-1,10 MM DMSO 溶液
- HA14-1,BCL-2抑制剂. BH-3 MIMETIC.
- REL-(ΑR,4R)-2-氨基-6-溴-4-(1-氰基-2-乙氧基-2-甲酰)-4H-苯并呋喃-3-羧酸
- 乙基-2-氨基-6-溴-4-(1-氰基-2-乙氧基-2-甲酰)-4H-苯并呋喃-3-羧酸
- 2-氨基-6-溴-ALPHA-氰基-3-(乙氧基羰基)-(ALPHAR,4R)-REL-4H-1-苯并吡喃-4-乙酸乙酯
- 4H-1-苯并吡喃-4-乙酸, 2-氨基-6-溴-Α-氰基-3-(乙氧基羰基)-, 乙酯, (ΑR,4R)-REL-
- 2-氨基-6-溴-ALPHA-氰基-3-(乙氧基羰基)-(ALPHAR,4R)-REL-4H-1-苯并吡喃-4-乙酸乙酯 1G
- 65673-63-4
- HA14-1, 10 mM in DMSO
- HA14-1, Bcl-2 inhibitor. BH-3 mimetic.
- HA14-1/HA141
- rel-Ethyl (αR,4R)-2-amino-6-bromo-α-cyano-3-(ethoxycarbonyl)-4H-1-benzopyran-4-acetate
- rel-(R)-Ethyl 2-amino-6-bromo-4-((R)-1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate(relative)
- HA14-1 USP/EP/BP
- 4H-1-Benzopyran-4-acetic acid, 2-amino-6-bromo-α-cyano-3-(ethoxycarbonyl)-, ethyl ester, (αR,4R)-rel-
- CS-392
- Ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-1-benzopyran-3-carboxylate
- HA14-1
- 2-AMINO-6-BROMO-ALPHA-CYANO-3-(ETHOXYCARBONYL)-4H-1-BENZOPYRAN-4-ACETIC ACID ETHYL ESTER
- ETHYL [2-AMINO-6-BROMO-4-(1-CYANO-2-ETHOXY-2-OXOETHYL)]-4H-CHROMENE-3-CARBOXYLATE
- ethyl (4S)-2-amino-6-bromo-4-[(R)-cyano-ethoxycarbonyl-methyl]-4H-chromene-3-carboxylate
- HA14-1;HA-14-1; HA 14-1
- Ethyl2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-1-benzopyran-3-carboxylate97%
- [Ethyl 2-amino-6-bromo-4-(1cyano-2-ethoxy-2-oxoethyl)-4H-chromene3-3carboxylate]
- HA 14-1 2-Amino-6-bromo-alpha-cyano-3-(ethoxycarbonyl)-(alphaR,4R)-rel-4H-1-benzopyran-4-acetic acid ethyl ester
- 2-Amino-6-bromo-alpha-cyano-3-(ethoxycarbonyl)-(alphaR,4R)-rel-4H-1-benzopyran-4-acetic acid ethyl ester HA 14-1
- (R)-ethyl 2-amino-6-bromo-4-((R)-1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate
- HA 14-1, Ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate, 2-[2-Amino-6-bromo-3-(ethoxycarbonyl)-4H-chromen-4-yl]-3-ethoxy-3-oxopropanenitrile
- Ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-1-benzopyran-3-carboxylate 97%
- 2-Amino-6-bromo-alpha-cyano-3-(ethoxycarbonyl)-(alphaR,4R)-rel-4H-1-benzopyran-4-acetic acid ethyl ester
- 2-Amino-6-bromo-α-cyano-3-(ethoxycarbonyl)-4H-1-benzopyran-4-aceticacidethylester
- [Ethyl 2-amino-6-bromo-4-(1cyano-2-ethoxy-2-oxoethyl)-4H-chromene3-3carboxylate], Ethyl [2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)]-4H-chromene-3-carboxylate
- 2-Amino-6-bromo-α-cyano-3-(ethoxycarbonyl)-4H-1-benzopyran-4-acetic acid ethyl ester, Ethyl [2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)]-4H-chromene-3-carboxylate
- ethyl 6-broMo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chroMene-3-carboxylate
- 2-Amino-6-bromo-alpha-cyano-3-(ethoxycarbonyl)-(4H)-1-benzopyrane-4-aceticacidethylester