ChemicalBook > CAS DataBase List > 16-HYDROXYHEXADECANOIC ACID
16-HYDROXYHEXADECANOIC ACID
16-HYDROXYHEXADECANOIC ACID
- CAS No.506-13-8
- Chemical Name:16-HYDROXYHEXADECANOIC ACID
- CBNumber:CB1488018
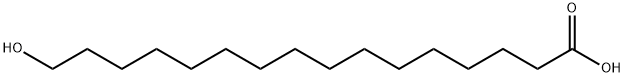
- Molecular Formula:C16H32O3
- Formula Weight:272.42
- MOL File:506-13-8.mol
16-HYDROXYHEXADECANOIC ACID Property
- Melting point 94-98 °C(lit.)
- Boiling point 414.4±18.0 °C(Predicted)
- Density 0.956±0.06 g/cm3(Predicted)
- storage temp. 2-8°C
- solubility DMF: 20 mg/ml; DMSO: 20 mg/ml; DMSO:PBS(pH 7.2) (1:2): 0.33 mg/ml; Ethanol: 2.5 mg/ml
- form Crystalline Powder
- pka 4.78±0.10(Predicted)
- color White
- BRN 1783998
- InChI InChI=1S/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19)
- InChIKey UGAGPNKCDRTDHP-UHFFFAOYSA-N
- SMILES C(O)(=O)CCCCCCCCCCCCCCCO
- LogP 4.904 (est)
- CAS DataBase Reference 506-13-8(CAS DataBase Reference)
- FDA UNII 7IPP3U0F3I
- EPA Substance Registry System 16-Hydroxypalmitic acid (506-13-8)
- UNSPSC Code 12352100
- NACRES NA.22
Safety
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
16-HYDROXYHEXADECANOIC ACID Price
More Price(4)
- Brand: Sigma-Aldrich(India)
- Product number: 177490
- Product name : 16-Hydroxyhexadecanoic acid
- Purity: 98%
- Packaging: 1G
- Price: ₹3344.93
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 177490
- Product name : 16-Hydroxyhexadecanoic acid
- Purity: 98%
- Packaging: 5G
- Price: ₹15977.7
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: H0675
- Product name : 16-Hydroxyhexadecanoic Acid
- Purity: min. 97.0 %
- Packaging: 1G
- Price: ₹5700
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: H0675
- Product name : 16-Hydroxyhexadecanoic Acid
- Purity: min. 97.0 %
- Packaging: 5G
- Price: ₹23400
- Updated: 2022/05/26
- Buy: Buy
16-HYDROXYHEXADECANOIC ACID Chemical Properties,Usage,Production
-
Description
16-
hydroxy Hexadecanoic acid is a metabolite of the saturated fatty acid palmitic acid (16:0) that has been hydroxylated on its terminal (ω) carbon. It is produced by ω- hydroxylation of palmitic acid by cytochrome P450 in both plants and animals. In plants, it is commonly a component of cutin. - Chemical Properties white crystalline solid
- Uses 16-Hydroxyhexadecanoic acid was used in the synthesis of dihydroxypalmitic acids. It was also used to induce the expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23.
-
Application
16-Hydroxyhexadecanoic acid can be used to:
(1) Oxidative reactions: Cell lysates and intact cells of Escherichia coli JM101 recombinant, which synthesises the cyp102 gene for cytochrome P450BM-3 monooxygenase, biotransform 16-hydroxyhexa-decanoic acid into 1, 16-hexadecanedioic acid. a small amount of intermediate product accumulation accompanies the oxidation of 16-hydroxyhexadecanoic acid. hydroxyhexadecanoic acid oxidation is accompanied by the accumulation of a small amount of intermediate products. The by-products also include 13.16-dihydroxyhexadecanoic acid and 12,16-dihydroxyhexadecanoic acid[1].
(2) 16-hydroxyhexadecanoic acid ethyl ester was synthesised from 16-hexadecanolactone by an intramolecular ester exchange reaction with PEG-lipase[2].
(3) Lactonisation reaction: It has been demonstrated that Mucor javanicus L46 and Mucor miehei catalyse the lactonisation reaction of 15-hydroxypentadecanoic and 16- hydroxyhexadecanoic acids to appropriate macrocyclic mono- and oligolactones[3].
- Definition ChEBI: 16-hydroxyhexadecanoic acid is an omega-hydroxy-long-chain fatty acid that is hexadecanoic acid (also known as palmitic acid) which is substituted at position 16 by a hydroxy group. It is a key monomer of cutin in the plant cuticle. It has a role as a plant metabolite. It is an omega-hydroxy-long-chain fatty acid and a hydroxypalmitic acid. It is a conjugate acid of a 16-hydroxyhexadecanoate.
-
References
[1] S. SCHNEIDER. Production Of Alkanedioic Acids By Cytochrome P450Bm-3 Monooxygenase: Oxidation Of 16-Hydroxyhexadecanoic Acid To Hexadecane-1, 16-Dioic Acid[J]. Biocatalysis and Biotransformation, 1999. DOI:10.3109/10242429909040113.
[2] YOH KODERA . Lactone synthesis from 16-hydroxyhexadecanoic acid ethyl ester in organic solvents catalyzed with polyethylene glycol-modified lipase[J]. Journal of biotechnology, 1993. DOI:10.1016/0168-1656(93)90162-G.
[3] U. ANTCZAK . Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones[J]. Enzyme and Microbial Technology, 1991. DOI:10.1016/0141-0229(91)90095-R.
16-HYDROXYHEXADECANOIC ACID Preparation Products And Raw materials
Raw materials
Preparation Products
Global(173)Suppliers
-
Supplier:
Finetech Industry Limited
- Tel:+86-27-8746-5837<br/>+8619945049750
- Email:info@finetechnology-ind.com
- Country:China
- ProdList:9642
- Advantage:58
-
Supplier:
Zhengzhou Anbu Chem Co.,Ltd
- Tel:+86-0371-88006763;<br/>+8615988602810
- Email:sales@anbuchem.com
- Country:China
- ProdList:2998
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
Shenzhen Nexconn Pharmatechs Ltd
- Tel:+86-755-89396905<br/>+86-15013857715
- Email:admin@nexconn.com
- Country:China
- ProdList:10406
- Advantage:58
-
Supplier:
Zhengzhou Alfa Chemical Co.,Ltd
- Tel: +8618530059196
- Email:sale04@alfachem.cn
- Country:China
- ProdList:12162
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
506-13-8, 16-HYDROXYHEXADECANOIC ACIDRelated Search:
- APOCHOLIC ACID BETULINIC ACID METHYL ESTER Sodium deoxycholate DIMETHYL HEXADECANEDIOATE 16-DOXYL-STEARIC ACID Sodium cholate DIHYDROOUABAIN Ursodeoxycholic acid LITHOCHOLIC ACID METHYL DESOXYCHOLATE HEXADECANEDIOIC ACID Betulinic acid Hyodeoxycholic acid Madecassoside 18β-Glycyrrhetinic Acid Maslinic acid NIGERICIN SODIUM SALT HYOCHOLIC ACID
- Organic Building Blocks
- Lipids
- Hydroxy Fatty Acids
- Fatty Acyls
- Fatty Acids and conjugates
- Chemical Synthesis
- Carboxylic Acids
- Carbonyl Compounds
- C13 to C42+
- Building Blocks
- Biochemicals and Reagents
- omega-Hydroxycarboxylic Acids
- omega-Functional Alkanols, Carboxylic Acids, Amines & Halides
- 抑制剂
- 烷基链连接子
- 化学试剂-有机试剂
- 高端化学
- 优势产品目录
- 有机化学
- 羧酸
- 羟基脂肪酸
- Organic Building Blocks
- Carboxylic Acids
- Carbonyl Compounds
- C13 to C42+
- Building Blocks
- 蠅-Hydroxycarboxylic Acids
- 蠅-Functional Alkanols, Carboxylic Acids, Amines & Halides
- HOCH215CO2H
- C16H32O3
- HOCH2CH214COOH
- 16-羟基十六碳酸
- 16-羟基十六烷酸
- 16-羟基十六酸, 97+%
- 16-羟基十六酸
- 杜松酸
- 16-羟基十六酸,98%
- 16-羟基棕榈酸
- 506-13-8
- 16-HYDROXYHEXADECANOI
- JUNIPERIC ACID
- 16-HYDROXYPALMITIC ACID
- 16-hydroxy-hexadecanoicaci
- junipericaci
- 16-HydroxyhexadecanoicAcid>
- 16-hydroxyhexadecanoate
- 16-Hydroxypalmitic acid, Juniperic acid
- 16-Hydroxyhexadecanoicacid,98%
- 16-Hydroxyhexadecanoic acid, 97+%
- 16-HYDROXYHEXADECANOIC ACID
- Hexadecanoic acid, 16-hydroxy-