ChemicalBook > CAS DataBase List > Oseltamivir
Oseltamivir
Description Indications Contraindications Dosage Interactions Mechanism of Action Pharmacodynamics Elimination Side Effects
Oseltamivir
- CAS No.196618-13-0
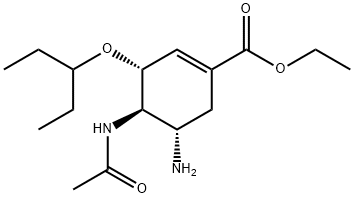
- Chemical Name:Oseltamivir
- CBNumber:CB1472402
- Molecular Formula:C16H28N2O4
- Formula Weight:312.4
- MOL File:196618-13-0.mol
Oseltamivir Property
- Melting point 107-108 °C
- Boiling point 473.3±45.0 °C(Predicted)
- Density 1.08±0.1 g/cm3(Predicted)
- storage temp. 2-8°C(protect from light)
- solubility Chloroform (Sparingly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly)
- pka 7.7 (25°); 6.6 (70°)
- form Solid
- color Off-White to Pale Beige
- Stability Hygroscopic
- CAS DataBase Reference 196618-13-0
- FDA UNII 20O93L6F9H
- NCI Dictionary of Cancer Terms Tamiflu
- NCI Drug Dictionary Tamiflu
- ATC code J05AH02
- EPA Substance Registry System 1-Cyclohexene-1-carboxylic acid, 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-, ethyl ester, (3R,4R,5S)- (196618-13-0)
Safety
- Hazardous Substances Data :196618-13-0(Hazardous Substances Data)
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P301+P312-P302+P352-P304+P340-P305+P351+P338
Oseltamivir Chemical Properties,Usage,Production
- Description Oseltamivir is a drug that suppresses the action of influenza A, influenza B, and H1N1 influenza viruses in children and adults. Oseltamivir is administered for the treatment of influenza (flu) for people who are above the age of 2 weeks and have been experiencing flu-like symptoms for less than two days. Oseltamivir may also be prescribed for people who are above 1-year-old as a protective measure to prevent them from contracting influenza due to exposure to the virus without necessarily having indicated any symptoms of infection. The drug may not treat common cold. Oseltamivir goes by the brand name Tamiflu.
- Indications Oseltamivir is prescribed for the treatment of mild or severe illness that results from infection with influenza A or B virus for patients above the age of 2 weeks, and have been experiencing flu-like symptoms for less than 48 hours. The drug is also used for preventive treatment against influenza amongst adolescents above 13 years and adults.
- Contraindications Oseltamivir is contraindicated in patients who may be hypersensitive to the medication or any of the ingredients in its formulation.
-
Dosage
For best results, Oseltamivir should be administered orally at least within two days of the onset of symptoms or exposure to influenza. The recommended dosage for treatment in adults with influenza is 74mg taken twice per day for 5 days.
In children, the recommended dosage should be 30-75mg (for children between 15kg and 40kg) take twice per day for 5 days, where dosing also depends on the body weight of the patient. For children who are 2 weeks old but <1 year, 3mg/kg should be administered orally two times per day.
For flu prevention in adults, 75mg of Oseltamivir should be administered on a daily basis for 10 days, whereas in children, 30-75mg should be given once per day for 10 days. -
Interactions
Drug interactions may influence the effectiveness of certain medications and may increase the risk of exposure to adverse side effects hence the need to consult a doctor with the list of other medications that one might be taking while getting a prescription for Oseltamivir.
A patient should notify their healthcare provider if they have received any nasal flu vaccines within 2 weeks before the administration of the first dose of Oseltamivir.
This drug may reduce protection against flu if the vaccine has been administered through the nose. One should wait for 2 weeks after the treatment with Oseltamivir before nasal administration of the flu vaccine. -
Mechanism of Action
The drug is an ethyl ester prodrug that necessitates hydrolysis for modification into the radical form, Oseltamivir carboxylate. The suggested mechanism of action of the drug is suppression of the influenza virus neuraminidase, which increases the probability of modification of the virus molecule, amalgamation and release.
Oseltamivir inhibits the spread of the influenza virus by preventing the action of neuraminidase, the enzyme that allows the spread of the virus from infected cells to those that are not infected. Oseltamivir inhibits the intercellular proliferation of the virus hence the duration and symptoms associated with influenza are also reduced. The length of the presenting symptoms may be reduced by one and a half days if treatment is initiated within 2 days at the onset of the flu symptoms. - Pharmacodynamics Oseltamivir is an antiviral medication, a neuraminidase suppressor that is used in prophylaxis and treatment of influenza A and B viruses. It is a prodrug that is prescribed as a phosphate, which is hydrolyzed hepatically into the radical metabolite, the unbound carboxylate of the drug (GS4071). Oseltamivir functions as a transitional analog suppressor of influenza neuraminidase.
- Elimination Upon primary absorption, >90% of Oseltamivir is eliminated by transformation into Oseltamivir carboxylate. The converted form of the drug does not undergo further metabolism, and it is excreted in urine. A significant portion, >99% of the Oseltamivir carboxylate is expelled from the body by renal excretion.
-
Side Effects
Common side effects associated with Oseltamivir include dizziness, headache, abdominal pain, bronchitis, diarrhea, vomiting, and nausea. Taking Oseltamivir after meals may help in reducing nausea. Other adverse side effects may include exacerbation of diabetes, behavioral disturbances, seizures, skin reactions, and allergic reactions.
A patient may need to seek emergency help if they are using Oseltamivir and they experience hallucinations, unusual behavior, shaking/tremors, and sudden confusion.
One should also consult their doctor if they are experiencing signs of an allergic reaction to the drug which may include hives, swelling in the throat or the face, difficulties in breathing, severe skin reactions such as a purple or red skin rash, sore throat, skin pain, peeling, burning eyes, and blistering. - Originator Tamiflu,Hoffmann-La Roche Inc
- Uses Oseltamivir is an orally active inhibitor of influenza virus neuraminidase; converted in vivo to the active acid metabolite. An antiviral drug. It is a COVID19-related research product.
- Definition ChEBI: A cyclohexenecarboxylate ester that is the ethyl ester of oseltamivir acid. An antiviral prodrug (it is hydrolysed to the active free carboxylic acid in the liver), it is used to slow the spread of influenza.
- Indications Oseltamivir phosphate (Tamiflu) is the ethyl ester prodrug of oseltamivir carboxylate, an analogue of neuraminic (sialic) acid that is a reversible competitive antagonist of influenza A and B neuraminidase.Influenza virus resistant to oseltamivir has not been found in naturally acquired isolates but has been isolated from influenza patients who have undergone treatment with this drug.These resistant strains contain mutations in the active site of neuraminidase and are generally less virulent and infective than nonresistant virus. In vitro passage of influenza virus in the presence of oseltamivir carboxylate can produce mutations in hemagglutinin that decrease the overall dependence of viral replication on neuraminidase; however, the clinical relevance of this resistance mechanism is unknown.
- Therapeutic Function Antiviral
- Antimicrobial activity Oseltamivir is active against influenza A and B, but no other virus.
- Acquired resistance Mutations in the neuraminidase (H274Y) have been detected in treated patients with seasonal H1N1 infection. Cross-resistance with zanamivir has been described in vitro.
- Pharmaceutical Applications A selective neuraminidase inhibitor, formulated as the phosphate salt of the ethyl ester for oral administration.
-
Pharmacokinetics
Oral absorption: c. 75%
Cmax 75 mg oral: 0.35–0.55 mg/L after 4 h
Plasma half-life: 7–9 h
Plasma protein binding: Not known
The ethyl ester prodrug is hydrolyzed by hepatic esterases to release the active compound, oseltamivir carboxylate. Drug is excreted in the urine as the carboxylate derivative. - Clinical Use Oseltamivir is approved for the treatment of uncomplicated acute influenza in patients aged 1 year and older. It decreases the duration of illness by 1 to 1.5 days when treatment is initiated within 48 hours of the onset of symptoms. Oseltamivir is also indicated for the prophylaxis of influenza in individuals aged 13 and older. It reduces infection rates to approximately 10 to 25% of that found in untreated populations; however, it is not intended to substitute for the early vaccination recommended by the CDC. Oseltamivir can be used as postexposure prophylaxis in household contacts of infected patients, with infection rates of treated patients around 10% of placebo control levels.
- Clinical Use Treatment and prevention of susceptible influenza A (H3N2) and B infections in adults and young children
- Side effects Adverse events relate to the gastrointestinal tract; the most common is nausea with or without vomiting in 10% of patients. Food alleviates side effects.
- Side effects The most frequently reported adverse effects of oseltamivir are nausea and vomiting.These events are usually mild to moderate, occur during the first 1 to 2 days of treatment, and can be lessened by taking the drug with food. Bronchitis, insomnia, and vertigo may also occur. Oseltamivir may not be indicated for use in certain individuals. Its efficacy in patients with chronic cardiac or respiratory disease has not been established. In clinical trials, no difference in the incidence of complications was seen between treatment and control groups. The efficacy of oseltamivir has not been demonstrated in immunocompromised patients, patients who begin treatment after 40 hours of symptoms, or patients given repeated prophylactic courses of therapy. Dosage adjustment is recommended for individuals with renal insufficiency; the drug’s safety in patients with hepatic insufficiency is unknown.
-
Metabolism
Oseltamivir is a prodrug; it is extensively metabolised by
esterases in the liver to the active carboxylate metabolite.
Oseltamivir carboxylate is not further metabolised and is eliminated entirely by renal excretion. Renal clearance exceeds glomerular filtration rate indicating that tubular secretion occurs in addition to glomerular filtration. Less than 20% of an oral radiolabelled dose is eliminated in faeces.
Oseltamivir Preparation Products And Raw materials
Raw materials
Preparation Products
Global(221)Suppliers
-
Supplier:
Anhui Ruihan Technology Co., Ltd
- Tel: +8617756083858
- Email:daisy@anhuiruihan.com
- Country:China
- ProdList:973
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Beijing Cooperate Pharmaceutical Co.,Ltd
- Tel:010-60279497
- Email:sales01@cooperate-pharm.com
- Country:CHINA
- ProdList:1803
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
Nanjing Dolon Biotechnology Co.,Ltd.
- Tel:18905173768
- Email:sales@dolonchem.com
- Country:CHINA
- ProdList:2972
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Casorganics US Corp
- Tel:+17326109938
- Email:sales@casorganics.com
- Country:CHINA
- ProdList:174
- Advantage:58
-
Supplier:
Shanghai Yingrui Biopharma Co.,Ltd
- Tel:21-33585366
- Email:export01@shyrchem.com
- Country:CHINA
- ProdList:1319
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
Related articles
196618-13-0, OseltamivirRelated Search:
- OseltaMivir iMpurity E Cefixime Cetirizine Acetaminophen Ribavirin Orlistat ZANAMIVIR HYDRATE Peramivir ETHYL(3R,4S,5S)-4,5-EPOXY-3-(1-ETHYL-PROPOXY)-CYCLOHEX-1-ENE-1-CARBOXYLATE(INTERMEDIATE OF OSELTAMIVIR PHOSPHATE ) OSELTAMIVIR PHOSPHATE,NTERMEDIATES OF OSELTAMIVIR Oseltamivir OSELTAMIVIR ACID-D3 OSELTAMIVIR-D3 PHOSPHATE OSELTAMIVIR MONO HYDROCHLORIDE OSELTAMIVIR ACID OSELTAMIVIR, [3H]- OSELTAMIVIR CITRATE
- Pharmaceutical
- 抑制剂
- 化学试剂
- WHO基本药物配体
- 生物医药-医药原料
- 医药体
- 医药原料
- 医用原料
- 原料药
- 原料
- 原料药及中间体
- 医药、农药及染料中间体
- 医药原料药
- 中间体
- 杂质对照品
- 对照品-杂质对照品
- 其他
- 有机中间体
- 微生物Microbiology
- C16H28N2O4
- 奥司他韦酸-13CD3杂质
- 乙腈中奥司他韦
- 甲醇中奥司他韦
- 奥司他韦检测标准品
- (3R,4R,5S)-4-乙酰氨基-5-氨基-3-(戊烷-3-基氧基)环己-1-烯甲酸乙酯(奥司他韦杂质)
- 奧司他韦
- 奧司他韦(BASE)
- (3R,4R,5S)-4-乙酰氨基-5-氨基-3-(戊-3-基氧基)环己-1-烯羧酸乙酯
- 奥司他韦 标准品
- 奥斯他韦中间体
- OSELTAMIVIR奥司他韦
- 奥司他韦磷酸酯
- (3R,4R,5S)-4-乙酰胺基-5-氨基-3-(1-乙基丙氧基)-1-环己烯-1-甲酸乙酯
- 达菲
- 奥司他韦(含磷酸盐)
- 奥塞米韦
- 奥司他韦
- 196618-13-0
- Oseltamivir 100 μg/mL in Acetonitrile
- Oseltamivr
- Ostavir
- (3R,4R,5S)-Ethyl 4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate (Oseltamivir Impurity)
- (-)-Oseltamivir
- (3R, 4R, 5S) -4-acetylamino-5-amino-3- (1-ethylpropoxy)-Ethyl 1-cyclohexene-1-formate
- (3R,4R,5S)-Ethyl 4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate
- TAMIFLU
- Ethyl (3R, 4R, 5S) - 4-acetylamino-5-amino-3 - (1-ethylpropoxy) - 1-cyclohexen-1-formate
- Oseltamivir D3Q: What is Oseltamivir D3 Q: What is the CAS Number of Oseltamivir D3 Q: What is the storage condition of Oseltamivir D3 Q: What are the applications of Oseltamivir D3
- OSELTAMIVIR USP/EP/BP
- 1-Cyclohexene-1-carboxylic acid, 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-, ethyl ester, (3R,4R,5S)-
- Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-pentan-3- yloxycyclohexene-1-carboxylate
- Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-(3-pentanyloxy)-1-cyclohexene-1-carboxylate phosphate
- Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate
- TaMvir
- TaMiflu-Free
- GS 4104
- GOP-A-Flu
- ethyl (3R,4R,5S)-5-aMino-4-acetaMido-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate