Uses and mechanism of Oseltamivir
Oseltamivir is the prodrug of oseltamivir carboxylate, a virustatic inhibitor of influenza A and B virus replication. Oseltamivir was developed by Gilead Sciences, Foster City, CA, USA, but it is marketed as Tamiflus by Roche Laboratories Inc. in most countries. The drug is available as a phosphate salt in capsules containing 30– 75 mg of the drug or as a powder that is reconstituted as an oral suspension. Oseltamivir was first approved in 1999.
Oseltamivir carboxylate belongs to the neuraminidase inhibitor (NAI) class of antiviral drugs. It selectively inhibits neuraminidase (NA)-mediated hydrolysis of host sialic acid residues bound to budding virions, a necessary step in influenza replication. Inhibition of NA blocks the release of progeny virions from infected cells, thereby impairing influenza virus replication and limiting influenza pathogenicity.
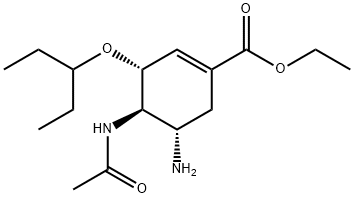
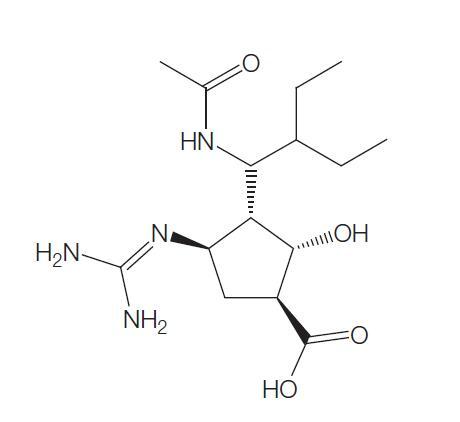
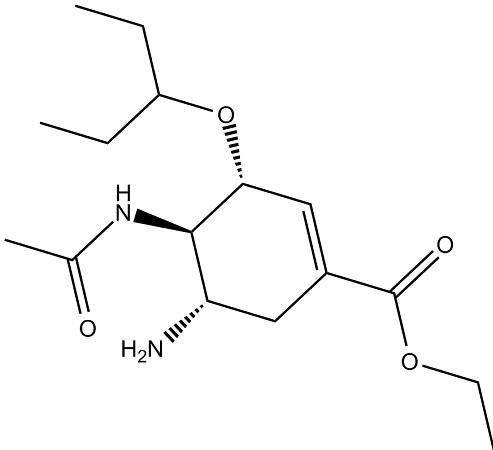
The chemical name of oseltamivir carboxylate is (3R, 4R, 5S)-4-
aetylamino-5-amino-3 (1-ethylpro-poxy)-1-cyclohexene-1-carboxylic
acid, ethyl ester phosphate); the chemical formula is C16H28N2O4
(free base) with molecular weight 312.4 and 410.4 (phosphate salt).
The structures of oseltamivir, the phosphate salt prodrug, and
oseltamivir carboxylate are as shown below.
Mechanism of action
Oseltamivir shares the same mechanism of action as zanamivir and peramivir as members of the NAI class of drugs, which, by definition, involves interference with influenza viral replication by inhibition of NA. Oseltamivir was designed as a carbocyclic transition state analog inhibitor of influenza NA with the expectation that it would retain the potency of zanamivir, the first NAI to be approved for clinical use, in a molecule that would be orally bioavailable. Oseltamivir carboxylate was comparable in potency and spectrum to zanamivir, but not more orally bioavailable. However, synthesis of its ester prodrug, oseltamivir ethyl phosphate, increased the bioavailability of oseltamivir carboxylate to 80% in humans and set the stage for its clinical development.
To inhibit NA, oseltamivir carboxylate with its bulky side chain must bind in the active enzyme site which is lined with 11 amino acids that are conserved among influenza viruses. Binding of the natural substrate sialic acid and its analog oseltamivir carboxylate in the site is competitive and occurs through specific interactions between these amino acids and portions of the sialic acid molecule or oseltamivir carboxylate molecules. For oseltamivir carboxylate to be accommodated in the enzymatic cleft requires that the glutamate at position 276 rotate and bond with an arginine residue at position 224, stabilized by binding to histidine at position 274. The resulting binding of oseltamivir carboxylate to NA interferes with its catalytic action and results in inhibition of viral replication. Parenthetically, any mutation that inhibits this rotation will reduce the binding affinity of oseltamivir carboxylate for the NA active site while still allowing binding of the natural substrate, sialic acid, and, also, NAIs that do not require this conformational change for binding, namely zanamivir.
Bioavailability
The pharmacokinetic profile of oseltamivir is simple and predictable. The absolute bioavailability of oseltamivir carboxylate is 80%. Following oral administration of doses from 50 to 500mg of the prodrug, oseltamivir ethyl ester, it is readily absorbed along the entire length of the small intestine. The drug undergoes extensive first-pass metabolism by hepatic and, perhaps, intestinal esterases that convert it to the antiviral metabolite oseltamivir carboxylate which can be detected in plasma within 30 min. Oseltamivir carboxylate peak concentrations (Cmax) in plasma are three times greater than those of oseltamivir ethyl ester levels and are reached in 3– 4 h. Plasma protein binding of the carboxylate is o3% and ethyl ester 42% over a wide range of concentrations.
Uses
Multiple double-blind, randomized clinical trials have shown that oseltamivir is efficacious and well tolerated for both prophylaxis and treatment of seasonal influenza in generally healthy individuals of 1 year of age and older. Similar results have been obtained by analysis of pooled data from treated high-risk elderly individuals (Z65 years; n=488) and a subset who had chronic respiratory and/or cardiac conditions (n=251). Similar analyses have demonstrated that oseltamivir treatment reduces the risk of respiratory tract complications requiring antibiotic therapy and or hospitalization.
Lastest Price from Oseltamivir manufacturers

US $2.00-6.00/kg2025-08-05
- CAS:
- 196618-13-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00-0.00/KG2025-04-21
- CAS:
- 196618-13-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20TONS