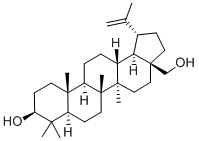
ChemicalBook > CAS DataBase List > Betulin
Betulin
Extract Physical and Chemical Properties Extraction method Identification method Physiological functions Storage References
Betulin
- CAS No.473-98-3
- Chemical Name:Betulin
- CBNumber:CB1301612
- Molecular Formula:C30H50O2
- Formula Weight:442.72
- MOL File:473-98-3.mol
Betulin Property
- Melting point 256-257 °C(lit.)
- alpha D15 +20° (c = 2 in pyridine)
- Boiling point 493.26°C (rough estimate)
- Density 0.9882 (rough estimate)
- refractive index 1.5045 (estimate)
- storage temp. 2-8°C
- solubility Chloroform (Slightly), Methanol (Slightly, Heated)
- form Off-white powder
- pka 15.10±0.10(Predicted)
- color Pale Brown to Beige
- optical activity +2015 (c 6, pyridine)
- Merck 14,1189
- Stability Hygroscopic
- InChIKey FVWJYYTZTCVBKE-ROUWMTJPSA-N
- LogP 8.607 (est)
- CAS DataBase Reference 473-98-3(CAS DataBase Reference)
- FDA UNII 6W70HN7X7O
- NIST Chemistry Reference Betulin(473-98-3)
- UNSPSC Code 85151701
- NACRES NA.77
Safety
- Hazard Codes :Xn,Xi
- Risk Statements :36/37/38-20/21/22-68/20/21/22-68
- Safety Statements :36/37-36-26
- WGK Germany :3
- RTECS :OK5755000
- HS Code :29181985
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H371
- Precautionary statements P260-P264-P270-P308+P311-P405-P501
Betulin Price
More Price(9)
- Brand: Sigma-Aldrich(India)
- Product number: B9757
- Product name : Betulin
- Purity: ≥98%
- Packaging: 1G
- Price: ₹17309.18
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: B9757
- Product name : Betulin
- Purity: ≥98%
- Packaging: 5G
- Price: ₹35116.3
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 92648
- Product name : Betulin
- Purity: analytical standard
- Packaging: 50MG
- Price: ₹11864.2
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 569371
- Product name : SREBP Processing Inhibitor, Betulin - CAS 473-98-3 - Calbiochem
- Purity: The SREBP Processing Inhibitor, Betulin, also referenced under CAS 473-98-3, controls the biological activity of SCAP (SREBP cleavage activating prote
- Packaging: 250MG
- Price: ₹8310
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: B0803
- Product name : Betulinol
- Purity: min. 97.0 %
- Packaging: 100MG
- Price: ₹7600
- Updated: 2022/05/26
- Buy: Buy
Betulin Chemical Properties,Usage,Production
-
Extract
Betulin is an extract from bark of the white birch tree, which has been known since the 18th century and is chemically defined. Betulin was discovered by German-Russian chemist Johann Tobias Lowitz. He was the first scientist to study and characterise betulin, and in doing was one of the first to isolate an active plant ingredient.
Betulin is a substance of pure plant origin that gives the birch bark its typical white colour. Betulin protects birch trees from environmental effects, such as extreme temperatures, pest infestations and solar radiation. - Physical and Chemical Properties It is white crystalline powder, soluble in alcohol, chloroform and benzene, slightly soluble in cold water, petroleum ether.
-
Extraction method
Currently Betulin is obtained mainly through direct extraction, and mainly by solvent reflux extraction and recrystallization purification
Specifically:
1. Extract betulin from ethanol solution. After heating reflux about 5h, the extract is vacuum distillated and recrystallized with ethanol for 2 to 3 times with ethanol to obtain crude product of betulin.
2. The crude product was recrystallized from methanol/chloroform (1: 1) (every 80g crude product requires 300mL methanol/chloroform solution).The mixture is allowed to stand overnight and then suction filtered to obtain white needle-like betulin.
3. Ultrasonic treatment for 20min when refluxing is carried out, and the extraction yield and product purity of betulin can be improved through the destruction of bark organization.
4. The optimum conditions of supercritical CO2 fluid extraction are as follows: the amount of modifier was 115mL/(g birch bark powder); extraction pressure is 20MPa; extraction temperature was 55 ℃; liquid CO2 flow rate was 10kg/h. -
Identification method
HPLC conditions for betulin:
Isolated Constituent: Betulin(10μl sample injection)
Column Stationary Phase: C18(150×2)mm 5μ
Column Mobile phase: Acetonitrile/water =30:70
Flow rate (ml/min);0.2Detection Wavelength (nm):270
Column Temperature:30℃ -
Physiological functions
- Betulin and its derivatives as biological agents has shown great potential in the treatment of HIV and cancer by interfering with the post-life cycle of the virus, which is related to the entry as well as growth and maturing of virus.
- As an effective anti-tumor drug, it can directly cause certain types of tumor cells to start self-destruction of the apoptosis program, and can slow down the growth of several types of tumor cells.
- It can reduce dietary induced obesity, reduce lipid content in serum and tissue and improve insulin sensitivity.
- It has mild anti-inflammatory properties at higher concentrations, and its anti-inflammatory properties are largely due to inhibition of non-neural gene pathways.
- Betulin in the body can inhibit the maturation of sterol regulatory element binding protein (SBERPs), thereby reducing the biosynthesis of cholesterol and fatty acid.
- In addition, with its anti-inflammatory, anti-virus effect and functions of inhibiting protein dissolution in the hair fiber, improving the luster of damaged hair and promoting hair growth and other activities, it can be used in food, cosmetics and pharmaceutical industries.
- Storage Cool and dry, kept from light and temperature.
- References http://www.imlan.de/en/infothek/what-is-betulin.html
-
Description
Sterol regulatory element binding protein 2 (SREBP-
2) regulates cholesterol synthesis by activating the transcription of genes for HMG- CoA reductase and other enzymes of the cholesterol synthetic pathway. When cellular sterol levels are high, SREBP is bound by SCAP and Insig to ER membranes as a glycosylated precursor protein. Upon cholesterol depletion, the protein is cleaved to its active form and translocated into the nucleus to stimulate transcription of genes involved in the uptake and synthesis of cholesterol. Betulin, the precursor of betulinic acid, is a pentacyclic triterpene found in the bark of birch trees. Betulin inhibits the SREBP- driven pathway of cholesterol and fatty acid biosynthesis by promoting SCAP–Insig binding which prevents the activation and release of SREBP- 2 from the ER. At 15- 30 mg/kg/day, betulin has been shown to decrease lipid levels and increase insulin sensitivity in mice fed a western- type diet. In an atherosclerosis disease model, 30 mg/kg/day betulin can reduce the size and improve the stability of atherosclerotic plaques in LDLR- knockout mice. At 2.5- 5 μg/ml betulin, in combination with cholesterol, demonstrates anticancer effects by inducing apoptosis in Jurkat cells, A549 lung carcinoma cells, and HeLa cervical carcinoma cells. - Chemical Properties crystals
- Uses birch bark extract is described as having anti-irritant and antiseptic properties, and effective in acne treatment. It is used to make sunburn products, soothing lotions, and aftershaves. The oil is astringent and is mainly used for its curative effects, especially in cases of acne and eczema. In folkloric medicine, birch bark extract was considered good for bathing skin eruptions. Destructive distillation of the bark’s white epidermis yields an empyreumatic oil known as oil of birch tar, Oleum rusci, or dagget. This is a thick, bituminous, brownish-black liquid with a pungent, balsamic odor. It contains a high percentage of methylsalicilate, creosol, and guaiacol and is almost identical to wintergreen oil.
- Uses antineoplastic, antihyperlipidemia
- Uses Betulin may be used in the preparation of betulinic acid, which shows anti-HIV, antimalarial and anti-inflammatory activities.
- Definition ChEBI: A pentacyclic triterpenoid that is lupane having a double bond at position 20(29) as well as 3beta-hydroxy and 28-hydroxymethyl substituents.
- General Description A cell-permeable pentacyclic triterpenoid from birch bark extract that interacts with SCAP (SREBP cleavage activating protein) and prevents SREBP (sterol regulatory element-binding protein) Golgi proteolytic activation (1 to 13.55 μM in Rat hepatocytes CRL-1601) via a similar mechanism as oxysterols by inducing the association of SCAP with Insig-1 (insulin-induced gene-1), thereby causing SCAP-SREBPs complex ER retention. Unlike oxysterols, Betulin does not activate LXR to induce concomitant HMGCR (HMG-CoA reductase) degradation and SREBP-1 up-regulation. While both Betulin and HMGCR inhibitor Lovastatin (Cat. No. 438185) inhibit cellular cholesterol synthesis (by ~70% in CRL-1601 cultures with respective compound at 13.55 and 1 μM concentration), only Betulin suppresses cellular fatty acid synthesis (by ~55% at 13.55 μM in CRL-1601). Betulin is shown to exhibit comparable in vivo efficacy as Lovastatin (both dosed at 30 mg/kg/day with chow) in ameliorate high fat/cholesterol diet-induced obesity (% fat/lean tissue ratio increase from non-fat diet mice = 167, 44, and 33 in mice consuming fat diet alone, with Lovastatin, with Betulin, respectively). However, only Betulin is demonstrated to lower fasting blood glucose and insulin levels and reduce fat cell size in white adipose tissue in high fat diet mice. Fatostatin (Cat. No. 341329) in comparison does not target SCAP via sterol-binding site, nor does it induce SCAP binding to Insig-1.
- Anticancer Research In addition, triterpenoids (betulin and its derivatives) isolated from HSSEactivated the signaling pathway regulated by p53 family genes, leading to the inhibition of BC cell viability or even the induction of apoptosis. And also, theseresearchers found that all the triterpenoids had no effect on normal breast cells.These findings provide an important basis for the use of those triterpenoids in thedevelopment of alternative therapies for breast cancer treatment (Hsu et al. 2015).
Betulin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(470)Suppliers
-
Supplier:
Hovane Phytochemicals Co., Ltd
- Tel:+86-17340288373
- Email:victor.liu@hovane.com
- Country:China
- ProdList:33
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-81139210<br/>+86-18192627656
- Email:1059@dideu.com
- Country:China
- ProdList:3003
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Kingfiner Technology Development Co.Ltd
- Tel:+86-15532196582<br/>+86-15373005021
- Email:lisa@kingfinertech.com
- Country:China
- ProdList:3005
- Advantage:58
-
Supplier:
Chongqing Zhihe Biopharmaceutical Co., Ltd.
- Tel:+86-18580541567<br/>+86-17782035140
- Email:sales@zhswyy.com
- Country:China
- ProdList:202
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
-
Supplier:
Shaanxi Xianhe Biotech Co., Ltd
- Tel: +8617709210191
- Email:Jerry@xhobio.com
- Country:China
- ProdList:884
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Nanjing Finetech Chemical Co., Ltd.
- Tel:025-85710122 17714198479
- Email:sales@fine-chemtech.com
- Country:CHINA
- ProdList:885
- Advantage:55
Related articles
473-98-3, BetulinRelated Search:
- Betulinic acid Resveratrol Rhynchophylline Lupeol ASTRAGALUS P.E. Bengenin Betulin-3-acetate,Betulin monoacetate Erythrodiol BETULIN DIACETATE(RG),BETULIN DIACETATE BETULINIC ACID METHYL ESTER 3BETA-ACETOXYBETULINIC ACID Betulin 3-epi-Betulin LUPANE betulin-(28)-5'-(aziridin-1-yl)-2',4'-dinitrobenzoate LUP-20(29)-EN-3,28-DIOL (BETULIN) betulin-(28)-5'-nitro-2'-furoate BETULIN(RG)
- 473-98-3
- standardized herbal extract
- reference standards from Chinese medicinal herbs (TCM).
- phytochemical
- pharmaceutical intermediate
- chemical reagent
- Pentacyclic Triterpenes
- natural product
- Tri-Terpenoids
- Triterpenoids
- 大化工产品
- 中药标准品,对照品
- 抑制剂
- 试剂
- 高端化学
- 高含量产品
- 有机产品
- 有机原料
- 化工原料
- 植物提取物单体
- 通用生化试剂-天然产物
- 化工材料
- 医药、农药及染料中间体
- 高含量单体
- 有机化学
- 标准品,对照品
- 分析试剂-对照品
- 三萜
- 标准品 -中药标准品
- 原料
- 标准品-中药标准品
- 植物成分单体
- 细胞生物学试剂
- 植物化学药
- 植物化学
- 标准产品
- 提取物
- 中药标准品
- 植物生化提取物
- 植提
- 保健品原料
- 有机锂化合物
- 中草药成分
- 五环三萜
- 医药原料
- 天然产物
- 植物提取物
- 对照品,标准品
- 标准品
- 中药对照品
- 对照品
- Complex Molecules
- Chiral Building Blocks
- Asymmetric Synthesis
- 桦木醇检测标准品
- 白桦脂醇/三萜类(TRITERPENOIDS)
- 桦皮脑
- (1R,3AS,5AR,5BR,7AR,9S,11AR,11BR,13AR,13BR)-3A-(羟甲基)-5A,5B,8,8,11A-五甲基-1-(丙-1-烯-2-基)二十氢-1H-环戊烷并[A]屈-9-醇