ChemicalBook > CAS DataBase List > RIBOSTAMYCIN SULFATE SALT
RIBOSTAMYCIN SULFATE SALT
RIBOSTAMYCIN SULFATE SALT
- CAS No.25546-65-0
- Chemical Name:RIBOSTAMYCIN SULFATE SALT
- CBNumber:CB1165751
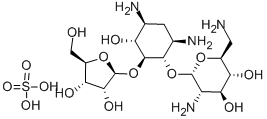
- Molecular Formula:C17H34N4O10
- Formula Weight:454.47
- MOL File:25546-65-0.mol
RIBOSTAMYCIN SULFATE SALT Property
- Melting point 192-195°; mp 175-180° (dec)
- alpha D23 +42°
- Boiling point 561.28°C (rough estimate)
- Density 1.1751 (rough estimate)
- refractive index 1.6700 (estimate)
- storage temp. 2-8°C
- pka 7.70(at 25℃)
- FDA UNII 2Q5JOU7T53
- ATC code J01GB10
Safety
- Hazard Codes :T
- Risk Statements :61-20/21/22
- Safety Statements :53-22-36/37/39-45
- WGK Germany :3
- RTECS :WK2300000
- Toxicity :mouse,LD50,intracrebral,66800ug/kg (66.8mg/kg),BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX)BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLDLUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION,Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 2489, 1970.
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
RIBOSTAMYCIN SULFATE SALT Chemical Properties,Usage,Production
- Description Ribostamycin was found in the culture broth of Streptomyces ribosidificus by Meiji Seika Kaisha in 1970 in the course of screening aminoglycoside antibiotics. It is structurally related to neomycin but lacks the diaminoidose (glucose) moiety substituted on the ribose moiety. Ribostamycin is much less toxic than neomycin and shows strong activity against a variety of gram-positive and gram-negative bacteria, except Pseudomonas aeruginosa. It is used parenterally for therapy of urinary tract, respiratory tract, surgical, and other infections.
- Uses Ribostamycin (Vistamycin) is a broad-spectrum aminoglycoside antibiotic. Ribostamycin is effective against Gram-Negative and Gram-Positive bacterial infection. Ribostamycin also inhibits the chaperone activity of PDI[1][2].
- Definition ChEBI: An amino cyclitol glycoside that is 4,6-diaminocyclohexane-1,2,3-triol having a 2,6-diamino-2,6-dideoxy-alpha-D-glucosyl residue attached at position 1 and a beta-D-ribosyl residue attache at position 2. It is an antibiotic produced by Streptomyces ribosidificus (formerly S. thermoflavus).
- Mechanism of action Ribostamycin (SF-733) is produced by Streptomyces ribosidificus. The free base is crystallized from methanol solution. The structure has been determined by chemical methods and total synthesis has been undertaken .
-
in vivo
Ribostamycin (40 mg/kg, intramuscular injection, per day for 14 days) causes little nephrotoxicity in rats(evaluated by urinalysis)[5].
- IC 50 Aminoglycoside
-
References
[1] Zheng T, et al. Linear self-assembly formation between gold nanoparticles and aminoglycoside antibiotics. Colloids Surf B Biointerfaces. 2018 Apr 1;164:185-191. DOI:10.1016/j.colsurfb.2018.01.027
[2] Hunfeld KP, et al. In vitro activity of mezlocillin, meropenem, aztreonam, vancomycin, teicoplanin, ribostamycin and fusidic acid against Borrelia burgdorferi. Int J Antimicrob Agents. 2001 Mar;17(3):203-8. DOI:10.1016/s0924-8579(00)00342-3
[3] Horibe T, et al. Ribostamycin inhibits the chaperone activity of protein disulfide isomerase. Biochem Biophys Res Commun. 2001 Dec 21;289(5):967-72. DOI:10.1006/bbrc.2001.6105
[4] Kong J, et al. Exploration of Antibiotic Activity of Aminoglycosides, in Particular Ribostamycin Alone and in Combination With Ethylenediaminetetraacetic Acid Against Pathogenic Bacteria. Front Microbiol. 2020 Jul 29;11:1718. DOI:10.3389/fmicb.2020.01718
[5] Kitasato I, et al. Comparative nephrotoxicity of ribostamycin and gentamicin in rats evaluated by urinalysis. Drugs Exp Clin Res. 1989;15(6-7):273-89. PMID:2591299
RIBOSTAMYCIN SULFATE SALT Preparation Products And Raw materials
Raw materials
Preparation Products
Global(28)Suppliers
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
-
Supplier:
Shaanxi Cuikang Pharmaceutical Technology Co., Ltd
- Tel: +8618791163155
- Email:18791163155@163.com
- Country:China
- ProdList:3558
- Advantage:58
- Supplier: Hangzhou Yuhao Chemical Technology Co., Ltd
- Tel:0571-82693216
- Email:info@yuhaochemical.com
- Country:China
- ProdList:9387
- Advantage:52
- Supplier: Beijing HuaMeiHuLiBiological Chemical
- Tel:010-56205725
- Email:waley188@sohu.com
- Country:China
- ProdList:12335
- Advantage:58
- Supplier: Zhejiang Huida Biotech Co., LTD
- Tel:0571-89903882<br/>15990081639
- Email:sunshixuan@huidabiotech.com
- Country:China
- ProdList:3644
- Advantage:58
- Supplier: cjbscvictory
- Tel:13348960310<br/>13348960310
- Email:3003867561@qq.com
- Country:China
- ProdList:10523
- Advantage:58
- Supplier: QUALITY CONTROL SOLUTIONS LTD.
- Tel:0755-66853366;<br/>13670046396
- Email:ORDERS@QCSRM.COM
- Country:China
- ProdList:24342
- Advantage:58
- Supplier: Zhejiang Huida Biotech Co., LTD
- Tel:0571-0571-89903882<br/>15990081639
- Email:sunshixuan@huidabiotech.com
- Country:China
- ProdList:3705
- Advantage:58
25546-65-0, RIBOSTAMYCIN SULFATE SALTRelated Search:
- 51797-35-6
- 抗生素 SF-733
- 25546-65-0
- ribomycin
- RIBOSTAMYCIN SULFATE SALT USP/EP/BP
- D-Streptamine, O-2,6-diamino-2,6-dideoxy-α-D-glucopyranosyl-(1→4)-O-[β-D-ribofuranosyl-(1→5)]-2-deoxy-
- vistamycin
- sf733
- ribostamycin
- hetangmycin
- d-ribofuranosyl-(1-5))-2-deoxy-
- 4-O-[2,6-Diamino-2,6-dideoxy-α-D-glucopyranosyl]-5-O-(β-D-ribofuranosyl)-2-deoxy-D-streptamine
- 4-O-(2,6-Diamino-2,6-dideoxy-α-D-glucopyranosyl)-5-O-(β-D-ribofuranosyl)-2-deoxy-D-streptamine
- Ribostamycin (base and/or unspecified salts)
- NSC 138925
- D-Streptamine, O-2,6-diamino-2,6-dideoxy-a-D-glucopyranosyl-(14)-O-[b-D-ribofuranosyl-(15)]-2-deoxy- (8CI, 9CI)
- dekamyciniv
- antibioticsf733
- RIBOSTAMYCIN SULPHATE
- RIBOSTAMYCIN SULFATE SALT
- RIBOSTAMYCIN DISULFATE