ChemicalBook > CAS DataBase List > Alitame
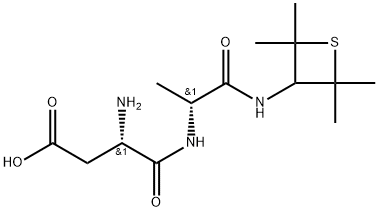
Alitame
Alitame
- CAS No.80863-62-3
- Chemical Name:Alitame
- CBNumber:CB0855163
- Molecular Formula:C14H25N3O4S
- Formula Weight:331.43
- MOL File:80863-62-3.mol
Alitame Property
- Melting point 136-147°
- alpha D25 +40 to +50° (c = 1 in water)
- Boiling point 608.5±55.0 °C(Predicted)
- Density 1.25±0.1 g/cm3(Predicted)
- solubility Methanol (Slightly)
- form Solid
- pka 3.71±0.10(Predicted)
- color White to Off-White
- Odor odorless
- Odor Type odorless
- LogP 1.508 (est)
- CAS DataBase Reference 80863-62-3
- FDA UNII PCE8DAE750
Safety
- HS Code :29400090
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302
- Precautionary statements P301+P312+P330
Alitame Chemical Properties,Usage,Production
- Chemical Properties White Solid
- Chemical Properties Alitame is a white nonhygroscopic crystalline powder; odorless or having a slight characteristic odor.
- Physical properties Alitame [L-a-aspartyl-N-(2,2,4,4-tetramethyl-3-thietanyl)-D-alaninamide] is a sweetener based on an amino acid. It is a very intense sweetener, possessing a sweetening power of about 2000 times that of sucrose. It also exhibits a clean sweet taste similar to sucrose. Although it is metabolized, so little is needed that its caloric contribution is insignificant. Alitame is prepared from the amino acids, L-aspartic acid, D-alamine, and a novel amine.
-
Characteristics
Alitame is an amino acidbased sweetener developed by Pfizer Central Research from l-aspartic acid, d-alanine, and 2,2,4,4-tetraethylthioethanyl amine. A terminal amide group instead of the methyl ester constituent of ASP was used to improve the hydrolytic stability. The incorporation of d-alanine as a second amino acid in place of l-phenylalanine has resulted in optimum sweetness. The increased steric and lipophilic bulk on a small ring with a sulfur derivative has provided a very sweet product and good taste qualities.
Alitame offers several benefits such as stability at high temperatures and a broader pH range. For instance, it is stable for over a year at pH 6–8 and room temperature and withstands pasteurization. However, prolonged storage of acidic solutions at high temperatures or in combination with certain ingredients (hydrogen peroxide or sodium bisulfite) may produce off-flavors. In the presence of high levels of reducing sugars, alitame can undergo Maillard reactions. - History Alitame, L-α-aspartyl-D-alanine N-(2,2,4,4-tetra methylthietan-3-yl)amide (5), was developed by Pfizer, but is owned by Danisco now. In 1986, Pfizer filed a food additive petition with the FDA. As of 2006, it was still pending. Alitame was approved for use as a sweetener by Australia in 1993, by China, Mexico, and New Zealand in 1994, by Indonesia in 1995, and by Colombia in 1996.
- Uses Non-nutritive sweetener.
- Uses Alitame is a dipeptide amide derivative of aspartic acid used as an artificial sweetener. Alitame is about ten times sweeter than Aspartame (A790015) with a half life about twice as long.
- Uses Alitame [L-a-aspartyl-N-(2,2,4,4-tetramethyl-3-thietanyl)-D-alaninamide] is a sweetener based on an amino acid. It is a very intense sweetener, possessing a sweetening power of about 2000 times that of sucrose. It also exhibits a clean sweet taste similar to sucrose. Although it is metabolized, so little is needed that its caloric contribution is insignificant. Alitame is prepared from the amino acids, L-aspartic acid, D-alamine, and a novel amine.
-
Production Methods
Alitame may be synthesized by a number of routes.For example,
3-(D-alaninamido)-2,2,4,4-tetramethylthietane is dissolved in water
and L-aspartic acid N-thiocarboxyanhydride is then added in
portions with vigorous stirring, maintaining the pH of 8.5–9.5.
The pH is then adjusted to 5.5 and p-toluenesulfonic acid
monohydrate is added over a period of one hour. The precipitated
crystalline p-toluenesulfonate salt is collected by filtration. To
obtain alitame from its salt, a mixture of Amberlite LA-1 (liquid
anion exchange resin), dichloromethane, deionized water, and the
salt is stirred for one hour, resulting in two clear layers. The aqueous
layer is treated with carbon, clarified by filtration, and cooled to
crystallize alitame.
Alternatively, tetramethylthietane amine is condensed with anNprotected form of D-alanine to give alanyl amide. This is then coupled to a protected analogue of L-aspartic acid to give a crude form of alitame. The crude product is then purified. - Definition ChEBI: Alitame is a dipeptide.
-
Pharmaceutical Applications
Alitame is an intense sweetening agent developed in the early 1980s
and is approximately 2000 times sweeter than sucrose. It has an
insignificant energy contribution of 6 kJ (1.4 kcal) per gram of
alitame.
Alitame is currently primarily used in a wide range of foods and beverages at a maximum level of 40–300 mg/kg. - Safety Profile The Joint Expert Committee on Food Additives (JECFA) concluded that alitame was not carcinogenic and did not show reproductive toxicity. In 1996, an ADI of 0–1 mg/kg of body weight was allocated. It is approved for use in Australia, New Zealand, Mexico, and China. A food additive petition was submitted to the FDA in 1986, and approval is awaited. In the petition, the estimated daily intake is 0.34 mg/kg of body weight, which represents the amount if alitame is the only sweetener in the diet. The level at which no observed adverse effects occur in animals is 100 mg/ kg. Potential uses include baked goods, baking mixes, hot and cold beverages, dry beverage mixes, tabletop sweeteners, chewing gum, candies, frozen desserts, and pharmaceuticals. Alitame has been approved for use in some countries such as Australia, Mexico, New Zealand, and China, but not in the United States or the EU.
-
Safety
Alitame is a relatively new intense sweetening agent used primarily
in foods and confectionary. It is generally regarded as a relatively
nontoxic and nonirritant material.
Chronic animal studies in mice, rats, and dogs carried out for a minimum of 18 months at concentrations >100 mg/kg per day exhibited no toxic or carcinogenic effects. In people, no evidence of untoward effects were observed following ingestion of 15 mg/kg per day for two weeks.
Following oral administration 7–22% of alitame is unabsorbed and excreted in the feces. The remaining amount is hydrolyzed to aspartic acid and alanine amide. The aspartic acid is metabolized normally and the alanine amide excreted in the urine as a sulfoxide isomer, as the sulfone, or conjugated with glucuronic acid.
The WHO has set an acceptable daily intake of alitame at up to 0.1 mg/kg body-weight.
LD50 (mouse, oral): >5 g/kg
LD50 (rabbit, skin): >2 g/kg
LD50 (rat, oral): >5 g/kg - Metabolism Alitame is noncariogenic. From an oral intake, 7%–22% is unabsorbed and excreted in the feces. The remainder is hydrolyzed to aspartic acid and alanine amide. The aspartic acid is normally metabolized, and the alanine amide is excreted in the urine as a sulfoxide isomer, sulfone, or conjugated with glucuronic acid. The incomplete absorption and metabolism result in a core value of 1.4 kcal g?1.
-
storage
Alitame is stable in dry, room temperature conditions but undergoes
degradation at elevated temperatures or when in solution at low
pH. Alitame can degrade in a one-stage process to aspartic acid and
alanine amide (under harsh conditions) or in a slow two-stage
process by first degrading to its β-aspartic isomer and then to
aspartic acid and alanine amide. At pH 5–8, alitame solutions at
238℃ have a half-life of approximately 4 years. At pH 2 and 238℃
the half-life is 1 year.
Alitame should be stored in a well-closed container in a cool, dry place. - Incompatibilities Alitame may be incompatible with oxidizing and reducing substances or strong acids and bases.
- Regulatory Status Alitame is approved for use in food applications in a number of countries worldwide including Australia, Chile, China, Mexico, and New Zealand.
Alitame Preparation Products And Raw materials
Raw materials
Preparation Products
Global(173)Suppliers
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Fengjia New Material Co., Ltd
- Tel:+86-0311-87836622<br/>+86-18712993135
- Email:sales01@tairunfaz.com
- Country:China
- ProdList:8051
- Advantage:58
-
Supplier:
Hebei Kingfiner Technology Development Co.Ltd
- Tel:+86-15532196582<br/>+86-15373005021
- Email:lisa@kingfinertech.com
- Country:China
- ProdList:3005
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
-
Supplier:
Frapp's ChemicalNFTZ Co., Ltd.
- Tel:+86 (576) 8169-6106
- Email:sales@frappschem.com
- Country:China
- ProdList:880
- Advantage:50
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Nanjing ChemLin Chemical Industry Co., Ltd.
- Tel:025-83697070
- Email:product@chemlin.com.cn
- Country:CHINA
- ProdList:3009
- Advantage:60
-
Supplier:
Nanjing Finetech Chemical Co., Ltd.
- Tel:025-85710122 17714198479
- Email:sales@fine-chemtech.com
- Country:CHINA
- ProdList:885
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
80863-62-3, AlitameRelated Search:
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Heterocycles
- Amino Acids & Derivatives
- 糖醇系列/天然/人工合成甜味剂
- 抑制剂
- 生化试剂
- 执行标准
- 威德利现货找张军
- 对照品
- 化学试剂
- 化工原料
- 营养强化剂
- 其他原料
- 非糖类甜味剂
- 甜味剂
- 食品添加剂
- C14H25N3O4S
- C14H25N3O4S25H2O
- L-Α-天冬氨酰-D-丙氨酰胺
- 阿丽甜
- 水中阿力甜溶液标准物质
- 水中阿力甜
- 阿力甜检测标准品
- ALITAME IN WATER/水中阿力甜
- 化合物 ALITAME ANHYDROUS
- 食品级阿力甜 阿力糖食用甜味剂
- 阿利甜
- 阿力甜科研试剂
- 化合物 T20165
- ≥99%阿力甜试剂
- 无水阿利坦
- 阿力甜(源头工厂)
- 水中阿力甜溶液,100ΜG/ML
- 同样无水的
- 西安食品级阿力甜
- 食品级阿力甜厂家直销
- 食品级阿力甜价格
- 食品级阿力甜用途
- 食品级阿力甜作用
- 食品级阿力甜厂家
- 食品级阿力甜生产厂家
- 食品级阿力甜
- 阿力甜价格
- 阿力甜用途
- 阿力甜作用
- 阿力甜厂家
- 阿力甜生产厂家
- 阿力甜 标准品
- 阿力甜
- 阿力甜溶液,100PPM
- L-天门冬酰-N-(2,2,4,4-四甲基-3-硫杂环丁基)-D-丙氨酰胺
- L-Α-天冬氨酰-N-(2,2,4,4-四甲基-3-硫化三亚甲基)-D-丙氨酰胺
- L-Α-天冬氨酰-D-丙氨酰胺
- L-Α-天冬酰-N-(2,2,4,4-四甲基-3-硫化三亚甲基)-D-丙胺酰胺(阿力甜)
- 80863-62-3
- (3S)-3-AMINO-4-([(1R)-1-METHYL-2-OXO-2-[(2,2,4,4-TETRAMETHYL-3-THIETANYL)AMINO]ETHYL]AMINO)-4-OXOBUTANOIC ACID
- Alitame