ChemicalBook > CAS DataBase List > Esfenvalerate
Esfenvalerate
Esfenvalerate
- CAS No.66230-04-4
- Chemical Name:Esfenvalerate
- CBNumber:CB0231185
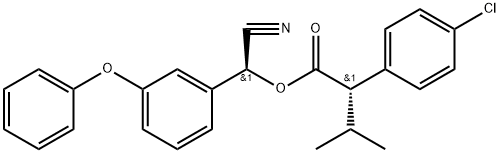
- Molecular Formula:C25H22ClNO3
- Formula Weight:419.91
- MOL File:66230-04-4.mol
Esfenvalerate Property
- Melting point 59°C
- alpha D25 -15.0° (c = 2.0 in CH3OH)
- Boiling point 151-167°C
- Density d2323 1.163
- vapor pressure 2×10-7 Pa (25 °C)
- refractive index 1.6500 (estimate)
- storage temp. 0-6°C
- solubility DMSO : 100 mg/mL (238.15 mM; Need ultrasonic)
- form Solid
- Water Solubility 0.002 mg l-1 (25 °C)
- color White to light yellow
- BRN 4275674
- LogP 6.220
- CAS DataBase Reference 66230-04-4(CAS DataBase Reference)
- FDA UNII 7F07OXM0PP
- NIST Chemistry Reference Benzeneacetic acid, 4-chloro-«alpha»-(1-methylethyl)-, cyano (3-phenoxyphenyl)methyl ester,(s-(r*,r*))-(66230-04-4)
- EPA Substance Registry System Esfenvalerate (66230-04-4)
- Pesticides Freedom of Information Act (FOIA) Esfenvalerate
- UNSPSC Code 41116107
- NACRES NA.24
Safety
- Hazard Codes :T;N,N,T
- Risk Statements :23/25-43-50/53
- Safety Statements :24-36/37/39-45-60-61
- RIDADR :UN 2811
- WGK Germany :3
- RTECS :CY1576367
- HazardClass :6.1(b)
- PackingGroup :III
- HS Code :29269090
- Hazardous Substances Data :66230-04-4(Hazardous Substances Data)
- Toxicity :LC50 (96-hour) for fathead minnows 0.69 mg/L (Worthing and Hance, 1991); acute oral LD50 for rats 75 mg/kg (Hartley and Kidd, 1987).
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H301+H331-H317-H410
- Precautionary statements P273-P280-P301+P310+P330-P302+P352-P304+P340+P311
Esfenvalerate Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 46277
- Product name : Esfenvalerate
- Purity: PESTANAL?, analytical standard
- Packaging: 100MG
- Price: ₹10099.73
- Updated: 2022/06/14
- Buy: Buy
Esfenvalerate Chemical Properties,Usage,Production
- Description Esfenvalerate is a synthetic pyrethoid insecticide – a class of pesticides originating from the chrysanthemum flower, which disrupt sodium channels. It has replaced fenvalerate, a less effective and more chronically toxic enantiomer. It can be used for general pest control, including spiders, fleas, scorpions, moths, beetles, etc. It is frequently applied on vegetable, nut, and non-crop products, often in combination with other active ingredients such as carbamate compounds and organophosphates. Product labels containing esfenvalerate include Sumi-Alpha, Hounddog, Sven, Asana, and Esfenvalerate 5EC, manufactured variously by BASF, Greencrop, and Standon.
-
Sources
http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/esfenvalerate-ext.html
https://en.wikipedia.org/wiki/Esfenvalerate
https://www.domyown.com/esfenvalerate-c-114_374.html
https://en.wikipedia.org/wiki/Pyrethroid
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/esfenvalerate
https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/269.htm - Chemical Properties Viscous yellow or amber liquid or white or amber crystalline solid.
- Uses Esfenvalerate is the most potent insecticidal isomer of Fenvalerate (F279450), a pyrethroid insecticide used to control insects from food crops, animal feed and cotton products.
- Uses Insecticide.
- Uses Esfenvalerate possesses a broad range of insecticidal activity and it is used on cotton, fruit, vegetables and other crops.
- Definition ChEBI: Esfenvalerate is a fenvalerate. It has a role as a pyrethroid ester insecticide and an agrochemical.
- Agricultural Uses Insecticide: A U.S. EPA restricted Use Pesticide (RUP). Esfenvalerate is a synthetic insecticide used to control a wide range of pests such as moths, flies, beetles, and other insects. It is used on vegetable crops (soya beans, sugar cane), tree fruit, cotton, maize, sorghum and nut crops, and non-crop lands. It also is used on a wide variety of household pests. It is usually mixed with a wide variety of other types of pesticides such as carbamate compounds or organophosphates and has the naturally occurring compound fenvalerate for use in the U.S. Esfenvalerate is almost identical to fenvalerate. Much of the data for fenvalerate is applicable to the pesticide esfenvalerate because the two compounds contain the same components. The only differences in the two products are the relative proportions of the four separate constituents (isomers). Esfenvalerate has become the preferred compound because it requires lower application rates than fenvalerate, is less chronically toxic, and is a more powerful insecticide.
- Trade name AMERICARE®; ASANA®; ASANA-XL®; ASANA® DPX-YB656-84; ENFORCER®; EVERCIDE®; HALMARK®; OMS-3023®; S-1844®; S-5602 ALPHA®; SUMI-ALFA®; SUMI-ALPHA®; SS-PYDRIN®; SUMICIDIN A-ALPHA®
- Potential Exposure Esfenvalerate is a synthetic pyrethroid Insecticide used to control wide range of pests such as moths, flies, beetles, and other insects. It is used on vegetable crops (soya beans, sugar cane), tree fruit, cotton, maize, sorghum and nut crops, and noncrop lands. It also isused on a wide variety of household pests. It is usually mixed with a wide variety of other types of pesticides such as carbamate compounds or organophosphates and has the naturally occurring compound fenvalerate for use in the United States Esfenvalerate is almost identical to fenvalerate. Much of the data for fenvalerate is applicable to the pesticide esfenvalerate (E:0207) because the two compounds contain the same components. The only differences in the two products are the relative proportions of the four separate constituents (isomers). Esfenvalerate has become the preferred compound because it requires lower applications rates than fenvalerate, is less chronically toxic, and is a more powerful insecticide. A United States Environmental Protection Agency Restricted Use Pesticide (RUP). Incompatibilities: Oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); strong acids. Moisture may cause hydrolysis/ decomposition.
- Environmental Fate Chemical/Physical. May hydrolyze in aqueous solutions forming acetic acid and other compounds.
- Metabolic pathway Esfenvalerate is the 2SαS-isomer of fenvalerate (which is racemic). It possesses some small differences in physical properties when compared with the racemate. Overall, the degradation and metabolism of the two compounds are very similar. Reference should be made to the fenvalerate entry. An important exception, which is discussed fully in the latter entry, is that esfenvalerate does not form the cholesterol ester conjugate that has been shown to be responsible for granulomatous changes in several tissues on repeated dosing of fenvalerate.
- Shipping UN3349 Pyrethroid pesticide, solid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous material. UN3352 Pyrethroid pesticide, liquid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
- Degradation Esfenvalerate is hydrolysed in aqueous alkali to its constituent acid and alcohol; the latter decomposes to 3-phenoxybenzaldehyde and cyanide ion as described for fenvalerate and cypermethrin. Photodecomposition occurs as for fenvalerate.
- Incompatibilities Oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); strong acids. Moisture may cause hydrolysis/ decomposition.
- Waste Disposal Incineration would be an effective disposal procedure where permitted. If an efficient incinerator is not available, the product should be mixed with large amounts of combustible material and contact with the smoke should be avoided. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers
Esfenvalerate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(167)Suppliers
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
SIMAGCHEM CORP
- Tel: +86-13806087780
- Email:sale@simagchem.com
- Country:China
- ProdList:17365
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Weifang Yuanye Biotechnology Co., Ltd
- Tel:15053681231 13354462013<br/>15053681231
- Email:tina@yuanyechemical.com
- Country:CHINA
- ProdList:67
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418684<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:25356
- Advantage:58
-
Supplier:
Hebei Fengjia New Material Co., Ltd
- Tel:+86-0311-87836622<br/>+86-18712993135
- Email:sales01@tairunfaz.com
- Country:China
- ProdList:8051
- Advantage:58
66230-04-4, EsfenvalerateRelated Search:
- 2-(4-Chlorophenyl)-3-methylbutyric acid (4-CHLOROMETHYLPHENYL)ACETIC ACID (3-Methoxyphenyl)acetonitrile Fenvalerate FEMA 2686 3-PHENOXYBENZALDEHYDE CYANOHYDRIN, 70 WT% SOLUTION IN ETHER 4-Chlorophenylacetic acid Esfenvalerate VALERATE Mandelonitrile ESFENVALERATE FREE ACID METABOLITE 2-HYDROXYPROPIONITRILE BENZYL PHENYLACETATE Methyl 4-chlorophenylacetate BENZYL-2-METHYLBUTYRATE ETHYL 4-CHLOROPHENYLACETATE Benzyl isobutyrate 3-PHENOXYPHENYLACETONITRILE
- Pyrethroids
- Pesticides&Metabolites
- Oeko-Tex Standard 100
- Insecticides
- EPA
- Endocrine Disruptors (Draft)Alphabetic
- E-GMethod Specific
- E
- Alpha sort
- PesticidesPesticides
- EQ - EZMethod Specific
- Insecticide
- 抑制剂
- 国家标准物质
- 高端化学
- 农残、兽药及化肥类
- 有机氯杀虫剂
- 杀虫(螨)剂
- 农药
- 拟除虫菊酯杀虫剂
- 分析标准品
- 杀虫剂
- C25H22ClNO3
- S-氰戊菊酯,10 MM DMSO 溶液
- 正己烷中顺式氰戊菊酯
- 正己烷中顺-氰戊菊酯溶液标准物质
- 正己烷中丙烯菊酯
- 正己烷/二氯甲烷中S-生物烯丙菊酯
- 乙腈中S-氰戊菊酯
- 甲醇中顺式氰戊菊酯
- 甲醇中S-氰戊菊酯溶液标准物质
- 甲醇中S-氰戊菊酯
- 甲苯中炔丙菊酯
- 丙酮中顺式氰戊菊酯
- 丙酮中S-氰戊菊酯溶液
- S-氰戊菊酯(顺式氰戊菊酯)
- S-氰戊菊酯(顺式氰戊菊酯 标准品
- 顺式氰戊菊酯,10NG/UL于环己烷 标准品
- 顺式氰戊菊酯(高效氰戊菊酯) 标准品
- S-氰戊菊酯溶液,1000PPM
- 正己烷中顺式氰戊菊酯-S-氰戊菊酯
- 正己烷中S-氰戊菊酯溶液
- 顺式氰戊菊酯(高效氰戊菊酯)
- 正己烷中顺式氰戊菊酯溶液标准物质
- 正己烷中顺式氰戊菊酯(S-氰戊菊酯)标准品
- (S)-2-氰基-3-苯氯苄基(S)-2-(4-氯基苯)-3-甲基丁酸酯
- (S)-α-氰基-3-苯氧苄基-(S)-2-(4-氯苯基)-3-甲基丁酸酯
- S-氰戊菊酯溶液,100PPM
- 双爱士,S-氰戊菊酯
- (S)-Α-氰基-3-苯氧苄基(S)-2-0(4-氯苯基)-3-甲基丁酸酯E
- 高效氰戊菊酯
- 高氰戊菊酯
- 顺式氰戊菊酯
- 双爱士
- 来福灵
- S-氰戊菊酯
- 顺式氰戊菊酯/高氰戊菊酯/(S)-2-氰基-3-苯氯苄基(S)-2-(4-氯基苯)-3-甲基丁酸酯
- 益化利