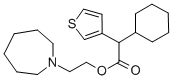
Originator
Stratene,Innothera,France,1973
Manufacturing Process
In a 100 ml flask fitted with a mechanical stirrer, a vertical condensor
protected by a calcium chloride stopper, a dropping-funnel and a source of
nitrogen were introduced 30 ml of hexamethylenephosphotriamide and 2.3 g
(0.1 mol) of finely cut sodium wire. A mixture of 12.3 g (0.1 mol) of (3-
thienyl)-acetonitrile and 16.3 g (0.1 mol) of cyclohexyl bromide was then
quickly added at a temperature of 20 C. The reaction mixture was then
maintained under nitrogen atmosphere and stirred for 12 hours at room
temperature. The excess of sodium was destroyed by adding 5 ml of ethanol
and the organic solution was slowly poured into 100 ml of a 1 N iced solution
of hydrochloric acid. The solution was extracted twice with 100 ml ether. The
ethereal phases were collected, washed with water, dried and concentrated
under reduced pressure. The crude product was then purified by chromatography on a silica column (150 g of silica) using a 1/1
benzene/cyclohexane mixture as elution agent. The product obtained was
rectified by distillation.
In this manner, 3.4 g of alpha(3-thienyl)-alpha-cyclohexylacetonitrile were
obtained, which represents a yield of 16%.
The nitrile may then be hydrolyzed to cyclohexyl-(3-thienyl)acetic acid which
is reacted with 1-(2-chloroethyl)-hexahydro-1H-azepine to give cetiedil. It is
commonly used as the citrate.