Manufacturing Process
(A) Preparation of 4-(3-Acetyl-4-Hydroxyphenyl)-2-Oxo-2,5-Dihydrofuran
(1567 CB): A solution of 57 grams of 4-(4-methoxyphenyl)-2-oxo-2,5-
dihydrofuran (0.3 mol) in 300 ml of methylene chloride is added slowly to 200
grams of anhydrous powdered aluminum chloride, while stirring and cooling in
a bath of iced water. When this is completed, one removes the bath and
leaves the reagents in contact for 10 minutes, and then introduces 72 grams
of acetyl chloride at a speed sufficient to maintain refluxing of the solvent.
One subsequently heats under reflux for 3 hours 30 minutes, decomposes by
pouring on to crushed ice, filters off the crystalline product and washes it with
water. 56 g, MP = 200°C. Yield: 80%. The product is recrystallized from acetic
acid and then melts at 201°-202°C.
(B) Preparation of 4-[3-Acetyl-4-(2-Oxopropyloxy)Phenyl]-2-Oxo-2,5-
Dihydrofuran: 5.45 grams (0.025 mol) of compound 1567 CB prepared
according to (A) dissolved in 50 ml of dimethyl formamide is stirred at room
temperature for 15 minutes with 5 grams of potassium carbonate and 1 gram
of sodium iodide, and 5 grams of chloracetone are then added drop by drop.
The temperature spontaneously rises a few degrees. The disappearance of the
phenolic compound is checked by testing with an alcoholic solution of ferric
chloride; this test should be negative at the end of the reaction
(approximately 2 hours). One then dilutes with 10 volumes of water, filters
the product which crystallizes out under these conditions and recrystallizes it
from acetic acid. It has the form of yellow needles (4 grams yield: 63%). MP,
= 155°-157°C.
(C) Preparation of 2-Acetyl-3-Methyl-5-(2-Oxo-2,5-Dihydro-4-
Furyl)Benro[b]Furan (3556 CB): (1) A suspension of 2 grams of the compound
prepared according to (B) in 20 ml of concentrated hydrochloric acid, is
heated to about 50°C, just until it dissolves. There after it is heated for 2
minutes to 70°C, just until precipitation commences. The mixture is allowed to
cool, diluted with water, filtered, the residue washed, dried, and sublimed at
200°C and 0.1 mm pressure. 1.4 grams of product (Yield: 70%) is obtained.
MPc=218°-221°C. A second sublimation produces a chemically pure product.
MPc= 221°-222°C. (2) Compound 1567 CB and chloracetone are caused to
react as in (B), the mineral salts subsequently filtered, 12 ml of concentrated
hydrochloric acid are added to the solution in dimethyl formamide without
dilution with water, and the mixture heated for 40 minutes on a water bath.
The product crystallizes in the warm mixture, the mixture is cooled to room
temperature, filtered, the residue washed with water and crystallized from
acetic acid. MPc= 222°C. Yield: 60% based on compound 1567 CB.
(D) Preparation of 2-(1-Hydroxyethyl)-3-Methyl-5-(2-Oxo-2,5-Dihydro-4-
Furyl)Benzo[b]-Furan (3574 CB): 13.2 grams of compound 3556 CB of which
the preparation is described in (C) are treated successively with 66 ml of
methylene chloride, 27 ml of methanol and, with stirring, 1.6 grams of sodium
borohydride added in stages. The reaction takes 1 hour. The mixture is poured
into water acidified with a sufficient amount of acetic acid, the solvents are
stripped under vacuum, the crystalline product removed, washed with water,
and recrystallized from ethyl acetate. Yield: 90%. MPk= 158°C.
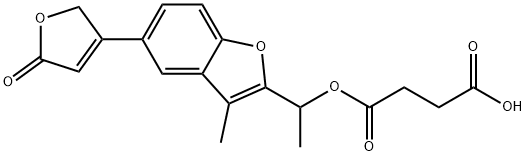
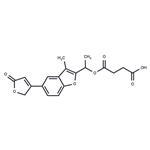
(E) Preparation of 2-(1-Succinyloxyethyl)-3-Methyl-5-(2-Oxo--2,5-Dihydro-4-Furyl)Benzo[b]-Furan (4091, CB): 8.65 grams of compound 3574 CB in 43 ml
of pyridine are warmed for 30 minutes, on a water bath, with succinic
anhydride. At the end of this, the pyridine is stripped off in vacuo. The
mixture is treated with dilute sulfuric acid and with ether, the crystalline
product filtered off, washed with water and with ether, and recrystallized from
ethyl acetate (9.35 grams). MPc=144°C (measured after drying at 90°C and
0.1 mm). Yield: 77%. The product yields an equimolecular compound with
morpholine. MPc=136°C (from ethyl acetate).