Manufacturing Process
A solution of 3-chloroperbenzoic acid (55%) in methylene chloride is cooled in
an ice bath. (5R)-(-)-Carvone are added dropwise thereto so that the
temperature does not rise above 20°C. The reaction mixture is stirred at room
temperature for 3.5 h, whereby a precipitate of 3-chlorobenzoic acid forms.
This suspension is stirred for 30 min and cooled with ice in order to complete
the precipitation. The precipitate is filtered off and washed with
hexane/methylene chloride (9:1). The filtrate is evaporated carefully. The
thus-obtained oil (epoxide) is suspended in ice-water and treated with 3 N
sulfuric acid while cooling in an ice bath so that the temperature does not rise
above 20°C. The mixture is stirred at room temperature for 15 h. The pH is
adjusted to 6.5 by adding 3 N sodium hydroxide solution. A small amount of
3-chlorobenzoic acid is filtered off. The aqueous phase is cooled to 0°C,
whereupon sodium (meta)periodate are added in portions over 30 min so that
the temperature does not rise above 20°C. After stirring at room temperature
for 2 h sodium sulfite and sodium bicarbonate are added in succession. The
suspension is filtered and the filtrate is extracted three times with methylene
chloride each time. The combined organic phases are dried over sodium
sulfate and evaporated. There is obtained (5R)-5-acetyl-2-methyl-2-
cyclohexen-1-one (crystallized from hexane/ethyl acetate at 0°C).
n-Butyltriphenylphosphonium bromide are suspended in dry tetrahydrofuran
under argon and cooled to -50°C. 1.33 N n-butyl lithium in hexane are added
thereto. The orange coloured suspension is warmed to room temperature and
then again cooled to -50°C. (5R)-5-Acetyl-2-methyl-2-cyclohexen-1-one
dissolved in dry tetrahydrofuran are added dropwise thereto over a period of
20 min. The cooling bath is then removed and the suspension is left to warm
to room temperature. After stirring for 2 h the reaction mixture is filtered
through siliceous earth. The filtrate is evaporated, the dark residue is
dissolved in ethyl acetate and washed twice with water each time. The organic
phase is dried over sodium sulfate and evaporated. The thus-obtained oil is
purified by column chromatography on silica gel with hexane/ethyl acetate
(95:5) as the elution agent. There is obtained (5R)-2-methyl-5-[(Z)-1-methyl-
1-pentenyl]-2-cyclohexen-1-one.
(5R)-2-Methyl-5-[(Z)-1-methyl-1-pentenyl]-2-cyclohexen-1-one are dissolved in methylene chloride and cooled to 0°C. After adding a catalytic amount zinc
chloride the solution is saturated with hydrogen bromide gas. After 30 min the
solution is filtered through silica gel and evaporated carefully. The resulting
oily residue is dissolved in dry ether, cooled to 0°C and treated with 100%
hydrogen peroxide. Silver trifluoroacetate dissolved in dry ether are added
dropwise over a period of 30 min. The excess of silver ions is precipitated by
adding 1 N hydrochloric acid and the suspension obtained is filtered through
silica gel. In order to remove the excess hydrogen peroxide, the filtrate is
washed 5 times with water each time. The ether phase is then evaporated
carefully and the residue is taken up methanol. A catalytic amount of sodium
methanolate is added to this solution, whereupon the mixture is left to stand
at room temperature for 15 h. The methanol is distilled off and the residue is
purified and separated into the two diastereomers (1:1) by chromatography
on silica gel while eluting with hexane/ethyl acetate (7:3). There are obtained
(1S,4R,5R,8S)-4-butyl-4,8-dimethyl-2,3-dioxabicyclo[3.3.1]nonan-7-one.
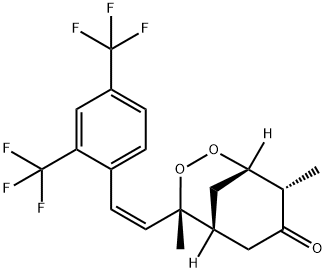
(1S,4R,5R,8S)-4-Butyl-4,8-dimethyl-2,3-dioxabicyclo[3.3.1]nonan-7-one is
dissolved in methylene chloride. 2,4-Bis(trifluoromethyl)
benzyltriphenylphosphonium bromide and sodium bis(trimethylsilyl)amide are
added thereto and the solution is left to stand at room temperature for 24 h.
The methylene chloride is distilled off and the (1S,4R,5R,8S)-4-[(Z)-2,4-
bis(trifluoromethyl)styryl-4,8-dimethyl-2,3-dioxabicyclo[3.3.1]nonan-7-one,
melting point 124°C is obtained.