Maximal allowable residue and maximal allowable usage amount
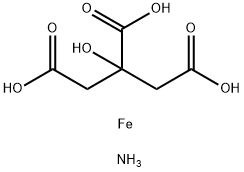
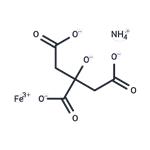
Name of additive: Ammonium ferric citrate [Mineral]
Function of additive: Nutrition Enhancer
Maximal allowable residue(g/kg): /
Food allowed to use it as additive
Maximal allowable amount(g/kg)
Salt, sandwich sugar
4000~8000mg (1: strengthened amount calculated on the element iron: cereal and its products 24~48mg/kg; beverage:10~20mg/kg dairy products; infant food 60~100mg/kg; sandwich sugar 600~1200 mg; 2. the iron content in various kinds of salts: ferrous sulfate (contains seven crystal water molecules), ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening.
High-iron cereals and their products (daily limit of such food: 50g)
1200~1350mg (1 the strengthening amount is calculated on the element iron: cereals and their products: 24~48mg/kg; drinks 10~20mg/kg; dairy products, infant food: 60~100mg/kg; sandwich sugar: 600~1200mg 2. The iron content in various kinds of iron salts ferrous sulfate (contains seven crystal water molecules), ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening.
Dairy products, infant food
400~800mg (1 the strengthening amount is calculated on the element iron: cereals and their products: 24~48mg/kg; drinks 10~20mg/kg; dairy products, infant food: 60~100mg/kg; sandwich sugar: 600~1200mg 2. The iron content in various kinds of iron salts ferrous sulfate (contains seven crystal water molecules), ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening.
Drink
70~140mg (1 the strengthening amount is calculated on the element iron: cereals and their products: 24~48mg/kg; drinks 10~20mg/kg; dairy products, infant food: 60~100mg/kg; sandwich sugar: 600~1200mg 2. The iron content in various kinds of iron salts ferrous sulfate (contains seven crystal water molecules), ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening.
Cereals and their products
160~330mg (1 the strengthening amount is calculated on the element iron: cereals and their products: 24~48mg/kg; drinks 10~20mg/kg; dairy products, infant food: 60~100mg/kg; sandwich sugar: 600~1200mg 2. The iron content in various kinds of iron salts ferrous sulfate (contains seven crystal water molecules), ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening.
Uses
1. Nutritional supplements. Iron enhancer, anti-anemia iron agent (each oral 1~2g). It can be added to biscuits, milk powder, being not suitable for being applied to food that is not colored.
2. Ammonium ferric citrate, as a food iron fortifier, has its absorption effect be better than inorganic iron. China provides that it can be used for salt and sandwich sugar with the usage amount of 4000~8000mg/kg; in high-iron cereal and its products (daily limit: 50 g of such food): 1200~1350mg/kg; dairy and infant food: 400 to 800 mg/kg; in cereals and their products, 160 to 330 mg/kg; beverages: 70 to 140 mg/kg.
3. It can be used for the pharmaceutical industry
4. It is used in the photographic industry and the pharmaceutical industry
5. It can be used as the nutritional supplements (iron fortifier). For dairy products, bread and wheat flour. Also used as anti-caking agent.
6. It can be used in bacterial culture medium for measuring aluminum, photography, blue print, blood and so on.
7. The brown product can be used as a hematinic drug for the treatment of iron deficiency anemia; it can be used as food additives; according to the provision of Japanese, "Food Additives", iron content: 16.5%-21.2%. The brown product and green product are both chemical-sensitive substances with the green goods being more sensitive to light than brown goods. Paper coated with green ferric ammonium citrate and red blood salt (potassium ferricyanide) is called blue blueprint paper. Upon the exposure, the trivalent iron in the citric acid salt is converted to bivalent, coming across water to give turnbull's blue, so the lightened part exhibits blue color while the rest part is still white, yielding a blue matrix white line pattern.
Production method
The ferric hydroxide is dissolved in citric acid, neutralized with ammonia solution, followed by drying to obtain it. (1) Preparation of ferric hydroxide Ferrous sulfate was added to the water, and sulfuric acid was slowly added thereto under stirring, followed by addition of an aqueous sodium chlorate solution. Then stir vigorously and raise the temperature to above 80 ℃; further add sodium chlorate. Stir until the reaction is terminated (no further ferrous reaction through ferricyanide test) to obtain the ferric sulfate solution. Add this solution to the reaction tank, add sodium hydroxide solution and stir vigorously with the reaction temperature of 80-90 °; when the reaction solution turns from viscous to thin, add water for washing until the sulfate and chlorine meet the requirements. Dry to get the iron hydroxide. (2) Preparation of ferric ammonium citrate; add citrate, ferric hydroxide and water into the reaction tank, stir and control the temperature at 95 ℃ above and maintain the temperature for 1h. And then cooled to 50?C and sent into ammonia under stirring. Stand for more than 48h. The supernatant was filtered and the filtrate was concentrated to a paste and dried at 80 ° C to obtain ferric ammonium citrate. The total yield of ferrous sulfate is 73-75%.
The same procedure as in preparation method 2 of brown ferric ammonium citrate.
Adding ammonium hydroxide to the ferric sulfate to generate ferrous hydroxide precipitate; filter and wash with water to no sulfate reaction anymore; then add citrate and heat to 60?C to completely dissolve the precipitate; neutralize with ammonia hydroxide and concentrate to a syrupy state, and coat on a glass plate, and dry to obtain a small sheet product.
Fe2 (SO4) 3 + 6NH3 + 6H2O → 3 (NH4) 2SO4 + 2Fe (OH) 3 ↓
2C6H8O7 + 3NH4OH + Fe (OH) 3 → (C6H5O7) 2Fe (NH4) 3 + 6H2O
It can also taken of ferrous sulfate as raw materials. 28g FeSO4 • 7H2O was dissolved in 4 mL water; add 3 mL 98% concentrated sulfuric acid and then drop 5mL 36% hydrogen peroxide; heat to 83 ℃ and stir for 0.5h; the blue color upon potassium ferricyanide test can tell the termination of the reaction; further add 100 mL distilled water, and 40% NaOH was added at 80 ° C with stirring to make the solution be alkaline. The precipitate was suction filtered and washed with hot water until no sulfate was present any more. The prepared Fe (OH) 3 was added to a citric acid solution (14 g/100 mL water) and stirred at 95 ° C for 1 h. The solution was then cooled to 50 ° C and an appropriate amount of aqueous ammonia was added. The solution was cooled to room temperature. Evaporate the supernatant to a paste with drying at 60~80 ° C to obtain the product with a yield of 91%.
A considerable amount of citric acid solution was added to ferric hydroxide produced from reaction between the ferric sulfate and aqueous ammonia; the concentrated slurry was coated on a glass plate, dried, and then peeled off from the glass plate.
Ferric hydroxide is dissolved in citric acid, with neutralization with ammonia hydroxide and evaporation at 60 ° C to obtain.
Hazards & Safety Information
Category: Toxic substances
Toxic classification: poisoning
Acute toxicity : Oral-rat LD:> 2000 mg/kg
Flammable and hazardous characteristics: being combustible with fire discharging iron and nitrogen oxide-containing spicy smoke
Storage and transport characteristics: Treasury: low temperature, ventilated, dry
Fire extinguishing agent : water, carbon dioxide, dry powder, sand
Occupational Standard: TWA 1 mg/m3
Description
Ammonium ferric citrate is a food additive with E number E381 used as an acidity regulator. It is a green or reddish-brown powder which is very soluble in water.
The molecular formula of ammonium iron (III) citrate is variable. It can be prepared by adding Fe(OH)3 to an aqueous solution of citric acid and ammonia . The brown form is approximately 9% NH
3, 16.5 – 18.5 % Fe , and 65 % hydrated citric acid; the green form is approximately 7.5 % NH3 , 14.5 – 16 % Fe, and 75% hydrated citric acid. The green type is more readily reduced by light than the brown.
Other uses for ammonium ferric citrate include water purification and printing (cyano type). It is used as a reducing agent of metal salts of low activity like gold and silver and is also in a commonly used recipe with potassium ferricyanide to make cyanotype prints. Ammonium ferric citrate is also used in Kligler iron deeps to determine hydrogen sulfide production in microbial metabolism.
In medicine, ammonium ferric citrate is used as a contrast medium. It is also used as a hematinic.
Chemical Properties
Ferric ammonium citrate forms reddish brown
flakes or grains, or a brownish-yellow powder. It has a
slight ammonia odor. There is also a green form that is
odorless.
Chemical Properties
brownish-yellow to brown powder
Chemical Properties
Ferric ammonium citrate (iron (III) ammonium citrate) is prepared by the reaction of ferric hydroxide with citric acid, followed by treatment with ammonium hydroxide, evaporating and drying. The resulting product occurs in two forms depending on the stoichi- ometry of the initial reactants. (1) Ferric ammonium citrate (iron (HI) ammonium citrate, CAS No. 1332-98-5) is a complex salt of undetermined structure composed of 16.5 to 18.5% iron, approximately 9% ammonia, and 65% citric acid and occurs as reddish brown or garnet red scales or granules or as a brownish-yellowish powder. (2) Ferric ammonium citrate (iron (HI) ammonium citrate, CAS No. 1333-00-2) is a complex salt of undetermined structure composed of 14.5 to 16% iron, approximately 7.5% ammonia and 75% citric acid and occurs as thin transparent green scales, as granules, as a powder, or as transparent green crystals. Supposedly, ferric ammonium citrate is one of the few soluble iron compounds which can be added to dairy products without inducing off-flavors.
Uses
Iron Ammonium Citrate is an anticaking agent used in salt. It is the chemical green ferric ammonium citrate.
Uses
Ferric Ammonium Citrate is a nutrient supplement that is a source of iron. It is prepared by the reaction of ferric hydroxide with citric acid, followed by treatment with ammonium hydroxide, evaporation, and drying. The resulting product occurs in two forms, i.e., brown with 16.5–18.5% iron and ferric ammonium citrate, green with 14.5–16% iron.
Uses
For blueprints; in photography.
General Description
Ammonium ferric citrate is a yellowish brown to red solid with a faint odor of ammonia. Ammonium ferric citrate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium ferric citrate is used in medicine, in making blueprints, and as a feed additive.
Air & Water Reactions
Water soluble.
Reactivity Profile
Acidic salts, such as Ammonium ferric citrate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Special Hazards of Combustion Products: Toxic oxides of nitrogen or ammonia gas may be formed in fires [USCG, 1999].
Health Hazard
Inhalation of dust irritates nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes and causes mild irritation of skin on prolonged contact.
Fire Hazard
Special Hazards of Combustion Products: Toxic oxides of nitrogen or ammonia gas may be formed in fires.
Flammability and Explosibility
Not classified
Potential Exposure
Ferric ammonium citrate is used in
blueprinting, photography, medical treatment; and as an
animal food additive.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Thesymptoms of metal fume fever may be delayed for 4-12 hfollowing exposure: it may last less than 36 h.Note to physician: In case of fume inhalation, treat pneumonitis. Give prednisone or other corticosteroid orally toreduce tissue response to fume. Positive-pressure ventilationmay be necessary. Treat metal fume fever with bed rest,analgesics, and antipyretics.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective in poisoning from iron.
storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from light.Sources of ignition, such as smoking and open flames, areprohibited where ferric ammonium citrate is used, handled,or stored in a manner that could create a potential fire orexplosion hazard.
Shipping
UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Compounds of the carboxyl group react
with all bases, both inorganic and organic (i.e., amines)
releasing substantial heat, water and a salt that may be
harmful. Incompatible with arsenic compounds (releases
hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides
(releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur).