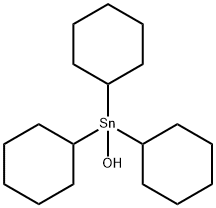
Description
Cyhexatin is a colorless to white, nearly odorless, crystalline powder. Molecular weight=385.16;Boiling point=227℃(decomposes); Freezing/Meltingpoint=195-198℃. Practically insoluble in water
Chemical Properties
Cyhexatin is a colorless to white, nearly odor-
less, crystalline powder.
Uses
Cyhexatin is used to control the motile stages of phytophagous
mites on pome and stone fruit, vines, hops, cotton, vegetables and
ornament ah.
Uses
Cyhexatin is a derivative of tricyclohexyltin and an effective acaricide used in the control of spider mites.
Definition
ChEBI: Cyhexatin is an organotin acaricide.
General Description
Technical Cyhexatin is a nearly odorless white crystalline powder that has no true melting point but degrades to bis(tricyclohexyl)tin oxide at 121 to 131°C which decomposes at 228°C; a melting point of 195-198°C is also reported. Very insoluble in water (less than 1 mg/L at 25°C), but wettable by water. Soluble in some organic solvents (acetone 1.3 g/L; xylenes 3.6 g/L; carbon tetrachloride 28 g/L; dichloromethane 34 g/L). Used as an acaricide (an agent to kill plant-feeding mites) in almonds, walnuts, hops and some fruits.
Reactivity Profile
Cyhexatin is incompatible with strong oxidizing agents. Soluble in some organic solvents (acetone 1.3 g/L; xylenes 3.6 g/L; carbon tetrachloride 28 g/L; dichloromethane 34 g/L). Stable in aqueous suspensions in neutral and alkaline pH (above pH 6), but reacts exothermically as a base in the presence of strong acids to form salts. Converts to dicyclohexyltin oxide and further to cyclohexylstannoic acid upon exposure to ultraviolet radiation.
Safety Profile
Poison by ingestion,
inhalation, and intraperitoneal routes.
Moderately toxic by skin contact.
Experimental reproductive effects. When
heated to decomposition it emits acrid
smoke and irritating fumes. See also TIN
COMPOUNDS.
Potential Exposure
Used as an agricultural chemical and
pesticide. A potential danger to those involved in the manu-
facture, formulation, and application of this acaricide
(miticide).
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Metabolic pathway
There is little published information of the degradation and metabolism
of cyhexatin. However, useful information on the fate of cyhexatin in a
water/sediment system can be deduced from published data on
azoc yclotin.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, dark,well-ventilated area away from oxidizers.
Shipping
UN2786 Organotin pesticides, solid, toxic,
Hazard Class: 6.1; Labels: 6.1-Poisonous material. UN2811
Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-
Poisonous materials, Technical Name Required.
Degradation
Cyhexatin is stable to hydrolysis at temperatures up to 100 °C from
slightly acid (pH 6) to alkaline conditions (PM).
Incompatibilities
Incompatible with strong oxidizers. May
react exothermically as base in the Incompatible with
strong oxidizers. May react exothermically as base in the
Incompatible with strong oxidizers. Reacts exothermically
as base in the presence of strong acids, forming salts. Keep away from ultraviolet radiation which may cause conver-
sion to dicyclohexyltin oxide and further to cyclohexylstan-
noic acid
.