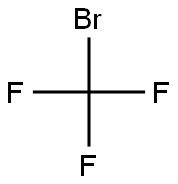
Chemical Properties
Trifluorobromomethane is a colorless gas with
a slight ethereal odor. Shipped as a liquefied compressed
gas.
Uses
Fire extinguishing agent; refrigerant.
Uses
Used as fire extinguishing agent for oil, electrical equipment, organic solvent, natural gas and a variety of organics, especially for important military and civilian sites.
Synthesis Reference(s)
Journal of the American Chemical Society, 68, p. 968, 1946
DOI: 10.1021/ja01210a017
General Description
A colorless, odorless gas at room conditions Shipped as a liquid confined under its own vapor pressure. Noncombustible. Nontoxic but can asphyxiate by the displacement of air. Contact with the unconfined liquid can cause frostbite by evaporative cooling. Exposure of the container to prolonged heat or fire can cause BROMOTRIFLUOROMETHANE to rupture violently and rocket.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
BROMOTRIFLUOROMETHANE may react with aluminum to produce substantial heat. Other halogenated hydrocarbons, such as fluorotrichloromethane, dichlorodifluoromethane, chlorodifluoromethane, tetrafluoromethane produce sufficient heat in this way to melt aluminum pieces. The vigor of the reaction appears to depend on the degree of fluorination and the vapor pressure [Chem. Eng. News 39(27):44 1961].
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Wildly toxic by
inhalation. Incompatible with aluminum.
When heated to decomposition it emits
toxic fumes of Fand Br-. See also
BROMIDES and FLUORIDES.
Potential Exposure
This material is used as a fire
extinguishing agent, a chemical intermediate, and as a
refrigerant.
Shipping
UN1009 Bromotrifluoromethane or Refrigerant
gas, R-13B, Hazard Class: 2.2; Labels: 2.2-Nonflammable
gas. Cylinders must be transported in a secure upright position,
in a well-ventilated truck. Protect cylinder and labels
from physical damage. The owner of the compressed gas
cylinder is the only entity allowed by federal law (49CFR)
to transport and refill them. It is a violation of transportation
regulations to refill compressed gas cylinders without
the express written permission of the owner.
Purification Methods
Purify the gas by passing it through a tube containing P2O5 on glass wool into a vacuum system where it is frozen out in a quartz tube and degassed by a cycles of freezing, evacuating and thawing. [Beilstein 1 III 83, 1 IV 73.]
Incompatibilities
Keep away from chemically active
metals, such as calcium, powdered aluminum; zinc, magnesium.
Attacks some plastics, rubber, and coatings.
Waste Disposal
Return refillable compressed
gas cylinders to supplier. Incineration, preferably after mixing
with another combustible fuel. Care must be exercised
to assure complete combustion to prevent the formation of
phosgene. An acid scrubber is necessary to remove the halo
acids produced.