Uses
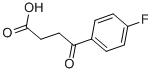
7-Fluoro-1-tetralone was prepared from 3-(4-fluorobenzoyl)-propionic acid by Wolff-Kishner reduction followed by ring closure with polyphosphoric acid and other substituted 1-tetralones where commercially available. Reduction of 3-(4-fluorobenzoyl)propionic acid with an excess of tert-butylamine borane (TBAB) in the presence of AlCl3 provided 4-(4-fluorophenyl)-1-butanol (87) in 63% yield for the synthesis of LM-1507 sodium salt. Penfluridol 1-[4,4-bis(4-fluorophenyl)butyl]-4-(4-chloro-3-trifluoromethylphenyl)- 4-hydroxypiperidine, C 28 H 27 ClF 5 NO, M r 523.99, mp 105 - 107°C, is obtained by reducing the ketone of 3-(4-fluorobenzoyl)propionic acid into a lactone.