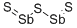
ANTIMONY(III) SULFIDE
- Product NameANTIMONY(III) SULFIDE
- CAS1345-04-6
- CBNumberCB9409860
- MFS3Sb2
- MW339.71
- EINECS215-713-4
- MDL NumberMFCD00011217
- MOL File1345-04-6.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 550 °C(lit.) | ||||||||||||||
| Boiling point | 1150 °C | ||||||||||||||
| Density | 4.64 g/mL at 25 °C(lit.) | ||||||||||||||
| refractive index | 4.303 | ||||||||||||||
| solubility | insoluble in H2O; soluble in concentrated HCl | ||||||||||||||
| form | powder | ||||||||||||||
| color | Dark gray to black | ||||||||||||||
| Specific Gravity | 4.64 | ||||||||||||||
| Water Solubility | Soluble in concentrated acids. Insoluble in water. | ||||||||||||||
| Crystal Structure | Sb2S3 type | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Merck | 14,713 | ||||||||||||||
| Space group | Pbnm | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
||||||||||||||
| Stability | Stable. Incompatible with acids (reacting to form poisonous hydrogen sulfide), moisture, water. | ||||||||||||||
| CAS DataBase Reference | 1345-04-6(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 1 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H351-H373-H412 | |||||||||
| Precautionary statements | P202-P260-P273-P280-P308+P313-P405 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 36/37/38-20/21/22 | |||||||||
| Safety Statements | 36/37/39-26 | |||||||||
| RIDADR | UN 1549 6.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | CC9450000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28309020 | |||||||||
| Hazardous Substances Data | 1345-04-6(Hazardous Substances Data) | |||||||||
| Toxicity | LD i.p. in rats: 100.0 mg Sb/100 g (Bradley, Fredrick) | |||||||||
| NFPA 704: |
|


