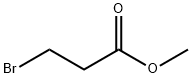
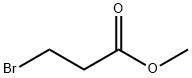
Chemical Properties
Very slightly yellow liquid
Uses
Methyl 3-bromopropionate was used in the synthesis of spiroanellated γ-lactones, pinacols and unprecedented 3-(1-hydroxycycloalkyl)-l-oxaspiro[
n,m]alkan-2-ones.
Synthesis
Methyl propiolate (52 mL, 0.583 mol) was mixed with recrystallized N-bromosuccinimide (120 g, 0.674 mol) in 1,700 mL of acetone under nitrogen protection. Pure silver nitrate (9.9 g, 0.0583 mol) was added to the solution in a single addition and the reaction mixture was stirred at room temperature for 6 hours. Upon completion of the reaction, the acetone was evaporated under reduced pressure (25 °C bath temperature) to give a gray slurry. The slurry was washed with hexane (2 x 200 mL), filtered to remove the gray solid, and the filtrate was concentrated under vacuum to give 95 g of a pale yellow oily crude product. The crude product was passed through a short distillation under reduced pressure (65 °C, ca. 25 mmHg) and the fractions were collected with a receiver cooled with dry ice/acetone to give 83.7 g (88% yield) of methyl 3-bromopropynoate as a pale yellow oil. Calculated values for elemental analysis of [C4H3BrO2]: C, 29.48; H, 1.86. Measured values: C, 29.09; H, 1.97.
References
[1] Patent: WO2004/13137, 2004, A1. Location in patent: Page 76
[2] Tetrahedron Letters, 2010, vol. 51, # 13, p. 1793 - 1796