Description
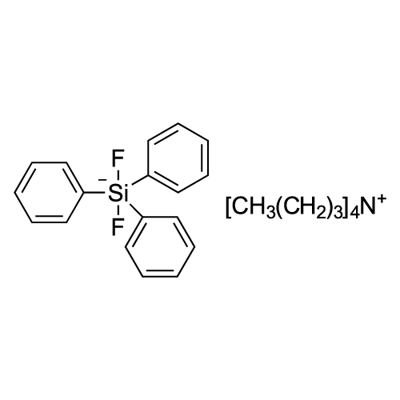
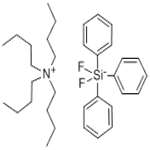
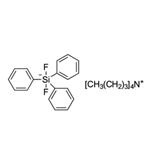
Tetrabutylammonium difluorotriphenylsilicate, also known as Tetrabutylammonium difluorotriphenylsilicate(IV) (TBADFTPS), is a fluoride source for nucleophilic fluorination. Tetrabutylammonium difluorotriphenylsilicate is an anion-exchange resin utilized in laboratory experiments for various scientific purposes. Its distinct characteristics render it useful for researchers in chromatography, catalysis, and electrochemistry. Tetrabutylammonium difluorotriphenylsilicate consists of two functional groups: a positively charged ammonium group and a negatively charged difluorotriphenylsilicate group. This resin allows for the exchange of anions within solutions. In chromatography and catalysis, Tetrabutylammonium difluorotriphenylsilicate effectively separates and purifies diverse compounds, including proteins, peptides, and organic molecules. Moreover, it catalyzes organic reactions and contributes to the synthesis of organic compounds. Additionally, Tetrabutylammonium difluorotriphenylsilicate plays a significant role in electrochemical experiments, aiding the study of electrochemical reactions and the measurement of electrochemical potentials.
Chemical Properties
Liquid
Physical properties
mp 155–156°C(EtOAc).
Uses
A fluoride source for nucleophilic fluorination.
Uses
Tetrabutylammonium Difluorotriphenylsilicate (Bardac(R) 208M) is a quaternary ammonium based antimicrobial used as a bacteriostat, deodorant, disinfectant and(or) a microbiocide.
Uses
Tetrabutylammonium Difluorotriphenylsilicate is non-hygroscopic, organic-soluble reagent used as a nucleophilic
fluoride source in Nucleophilic Fluorination and Allylation Reactions.
Preparation
Tetrabutylammonium Difluorotriphenylsilicate is prepared by treatment of triphenylsilyl fluoride
with tetrabutylammonium fluoride (TBAF), or by reaction
of triphenylsilane with tetrabutylammonium hydrogen
difluoride.