Description
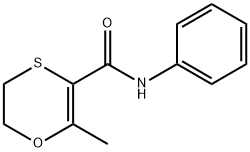
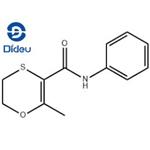
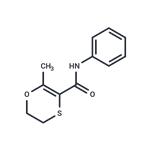
Carboxin is a white crystalline solid.Molecular weight=235.32; Freezing/Meltingpoint=91.5-95℃ (depending on crystal structure); Vaporpressure=1.78 x 10 2 7 mmHg. Practically insoluble inwater; solubility=25 mg/L; 0.15 g/L at 20℃.
Chemical Properties
Carboxin is a white crystalline solid
Uses
Carboxin is used as a seed treatment for cereals and as a seedling
treatment on many cereals, beans, and vegetable crops and cotton. It is
also used for the treatment of turf.
Uses
Systemic plant fungicide.
Uses
Carboxine is an fungicide used for the control of fruit rot of custard apple.
Definition
ChEBI: An anilide obtained by formal condensation of the amino group of aniline with the carboxy group of 2-methyl-5,6-dihydro-1,4-oxathiine-3-carboxylic acid. A fungicide for control of bunts and smuts normally that is normally used as a seed treatment.
General Description
Off-white crystals. Systemic fungicide and seed protectant.
Agricultural Uses
Fungicide: Carboxin is a General Use Pesticide (GUP) and is used as a seed protectant. It is often used in combination with other fungicides such as thiram or captan. Carboxin is a systemic anilide fungicide. It is used as a seed treatment for control of smut, rot, and blight on barley, oats, rice, cotton, vegetables, corn and wheat. It is also used to control fairy rings on turf grass. Carboxin may be used to prevent the formation of these diseases or may be used to cure existing plant diseases. Also used as a wood preservative.
Trade name
CADAN®; CARBOXIN OXATHION PESTICIDE®; CASWELL No. 165 A®; D-735®; F-735®; FLO PRO V SEED PROTECTANT®[C]; KEMIKAR®; OXALIN®; PADAN®; SANVEX®; THIOBEL®; VEGETOX®; VITAFLO®; VITAVAX® 200FF; V 4X®
Safety Profile
Poison by ingestion.
Moderately toxic by skin contact and
possibly other routes. Mutation data
reported. When heated to decomposition it
emits very toxic fumes of NOx and SOx.
Potential Exposure
A potential danger to those involved
in the production, Formulation and application of this systemic fungicide, seed protectant and wood preservative
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Biological. The sulfoxidation of carboxin to carboxin sulfoxide by the fungus Ustilago
maydis was reported by Bollag and Liu (1990).
Soil. Carboxin oxidized in soil forming carboxin sulfoxide. The half-life in soil was
reported to be 24 hours (Worthing and Hance, 1991).
Plant. In plants (barley, cotton and wheat) and water, carboxin oxidizes to the corresponding sulfoxide (Worthing and Hance, 1991).
Metabolic pathway
Carboxin is a systemic fungicide which is very stable to hydrolysis but
is readily oxidised at sulfur to a sulfoxide and a sulfone. The latter,
oxycarboxin, is itself a commercial fungicide. Metabolism is mainly
by oxidation at sulfur in soil, plants and animals but hydroxylation of
the phenyl ring is also important in animals. Hydrolysis has been
convincingly demonstrated only in plants (peanut).
storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with carboxin youshould be trained on its proper handling and storage. Storein tightly closed containers in a cool, well-ventilated area
Shipping
UN2588 Pesticides, solid, toxic, Hazard Class: 6.1;
Labels: 6.1-Poisonous materials, Technical Name Required.
Degradation
Carboxin is stable to hydrolysis (25 °C) at pH 5,7 and 9. Measurable rates
are seen only at higher pH and occur by nucleophilic attack by hydroxyl
ion at carbonyl. The half-life in 0.5 N NaOH is 107 days. Thus chemical
hydrolysis is not expected to be significant under environmental conditions
(El-Dib and Aly, 1976).
The compound is very labile to aqueous photolysis with a DT
50 of
<3 hours (PM).
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. Avoid heat and humidity. Thermal decomposition products may include cyanide gas and cyanide salts.
Waste Disposal
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal, state,
and local environmental regulations must be observed.