Carboxin: Biodegradation and Preparation Method
General Description
Carboxin is a fungicide widely utilized in agriculture for its effectiveness against various fungal diseases in crops. Its mechanism involves inhibiting fungal growth by disrupting cell wall synthesis, minimizing harm to beneficial organisms and plants. However, careful management is essential to prevent environmental or produce toxicity. Biodegradation studies have isolated a strain, Delftia sp. HFL-1, capable of efficiently degrading carboxin and its metabolite aniline. Optimal conditions for degradation include temperatures of 30-42 °C and pH levels of 5-9. Molecular analysis revealed a degradation pathway involving hydrolysis into aniline, further conversion to catechol, and eventual mineralization. The preparation method involves specific steps and catalysts to achieve a high yield of 69%, emphasizing sustainability through reusing reagents.

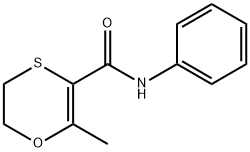
Figure 1. Carboxin
Overview
Carboxin is a widely used fungicide in agricultural practices, prized for its effective control against a range of fungal diseases in various crops. Its mode of action lies in inhibiting the growth and reproduction of fungi by disrupting their cell wall synthesis. The chemical's specificity towards fungal cells minimizes harm to beneficial microorganisms and plant tissues. However, its use must be carefully managed to avoid residual toxicity in the environment or on produce. Overall, carboxin represents a valuable tool in sustainable agriculture, balancing disease control with environmental stewardship. 1
Biodegradation
Carboxin, a fungicide commonly used for seed coating to prevent plant diseases, can lead to soil pollution due to its direct entry into the soil along with seeds. In a study focusing on bioremediation, a novel carboxin-degrading strain called Delftia sp. HFL-1 was isolated. This strain demonstrated the ability to utilize carboxin as a carbon source for growth and efficiently degrade both carboxin and its metabolite aniline within 24 hours. The optimal conditions for carboxin degradation by strain HFL-1 were found to be temperatures ranging from 30 to 42 °C and pH levels between 5 and 9. Through High-Resolution Mass Spectrometer analysis, the complete mineralization pathway of carboxin by strain HFL-1 was elucidated. Carboxin was initially hydrolyzed into aniline, which was further metabolized into catechol through oxidation processes. Eventually, it was converted into 4-hydroxy-2-oxopentanoate, a precursor of the tricarboxylic acid cycle. Genome sequencing revealed specific degradation genes and clusters related to carboxin degradation, including key enzymes like amidohydrolase and dioxygenase. The discovery of transposons suggested that the aniline degradation gene cluster in strain HFL-1 was acquired through horizontal transfer. Moreover, the identified degradation genes were cloned and overexpressed, demonstrating their efficiency in degrading aniline in vitro. This research provides valuable insights into the molecular mechanisms underlying the biodegradation of carboxin and its metabolite aniline, offering a promising approach for the bioremediation of contaminated soil. 2
Preparation method
The preparation method of Carboxin involves several steps with specific reagents, solvents, and catalysts to achieve a high yield of 69%. To begin, the process starts by reacting acetoacetanilide with bis(2-hydroxyethyl) disulfide in a sodium hydroxide (NaOH) solution. This reaction produces an intermediate compound, anilide of 2-(2-hydroxyethylthio)acetoacetic acid. The next step involves extracting this intermediate compound using toluene as the solvent. Following the extraction, the intermediate compound undergoes cyclization to form Carboxin. This cyclization process is carried out in the presence of p-toluenesulfonic acid as a catalyst. The use of the catalyst is crucial for facilitating the cyclization reaction efficiently. Moreover, bis(2-hydroxyethyl) disulfide, which is recovered from the aqueous residue in the initial step, is regenerated and reused in the reaction, ensuring a more sustainable and efficient synthetic process. Throughout the preparation method of Carboxin, specific temperature conditions are maintained, ranging from room temperature to elevated temperatures, and different reaction durations are applied at various stages to optimize the synthesis process and achieve the desired product yield. 3
Reference
1. Carboxin. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 21307.
2. Li S, Geng Y, Bao C, et al. Complete biodegradation of fungicide carboxin and its metabolite aniline by Delftia sp. HFL-1. Sci Total Environ. 2024; 912: 168957.
3. Wyrzykowska U. Strzelecki M. Sidorczuk W. Method of preparing 5,6-dihydro-2-methyl-N-phenyl-1,4-oxathiin-3-carboxamide. 2008; Patent Number: PL198675.
Related articles And Qustion
Lastest Price from Carboxin manufacturers

US $0.00/kg2025-04-11
- CAS:
- 5234-68-4
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 20tons
US $0.00/kg2025-03-03
- CAS:
- 5234-68-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS