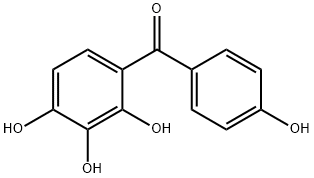
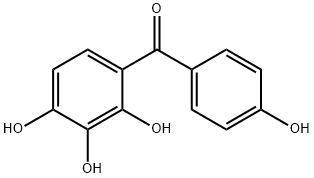
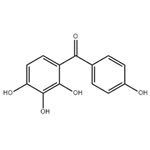
Chemical Properties
Yellow powder
Uses
2,3,4,4'-Tetrahydroxybenzophenone can be used for positive resist composition.
Preparation
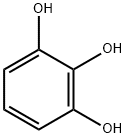
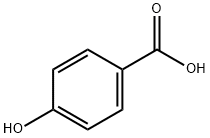
Preparation by benzoylation of pyrogallol with p-hydroxybenzoic acid in the presence of Amberlyst-15 in refluxing toluene for 21 h under azeotropical removal of water (86%).
Synthesis
General procedure for the synthesis of (4-hydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone from o-hydroxybenzenetriol and p-hydroxybenzoic acid: 0.11 moles of p-hydroxybenzoic acid and 0.1 moles of pyrophosphoric acid as an acylating agent as well as 0.015 moles of concentrated sulfuric acid were added in a reactor and mixed well. An inert gas was passed for 10 minutes to remove air. After increasing the system temperature to 70-80°C, the reactor was closed, the temperature was adjusted to 110-130°C, the pressure was maintained at 0.1-5 MPa and the reaction was carried out for 3 hours. Upon completion of the reaction, the reaction mixture was cooled to 50-60 °C and held at this temperature for 1.5 hours. Subsequently, 200 ml of 20% ethanol solution was added and crystallized at 0-5°C. The crystals were collected by filtration and dried to give the target product (4-hydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone. The molar yield of this step was 78.16% and the purity was ≥98.5% by HPLC.
References
[1] Patent: CN106349036, 2017, A. Location in patent: Paragraph 0032-0037
[2] Patent: CN106365961, 2017, A. Location in patent: Paragraph 0030; 0031; 0032; 0033; 0034; 0035
[3] Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 2, p. 482
[4] Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 2, p. 483